NDC Code(s) : 48102-007-35, 48102-007-11, 48102-007-13
Packager : Fera Pharmaceuticals
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| BACITRACINBACITRACIN OINTMENT | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
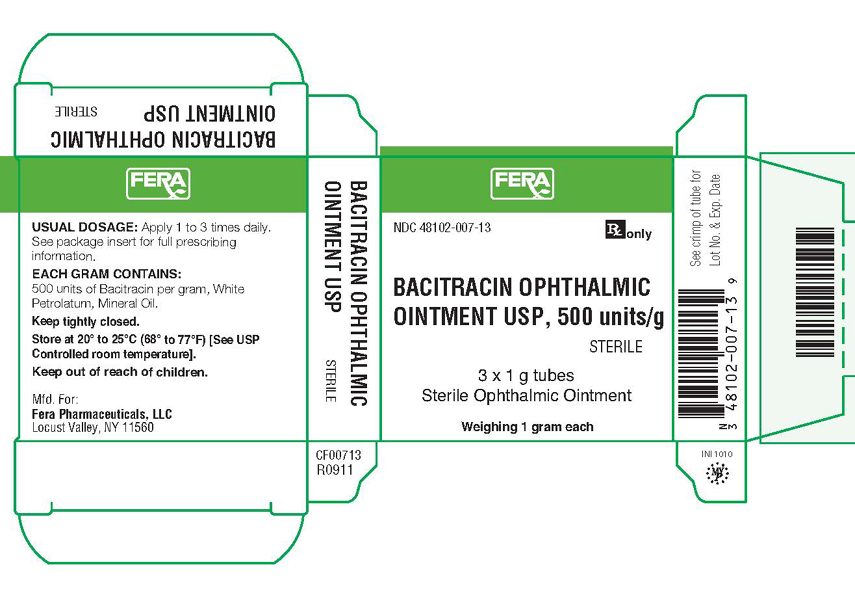
NDC 48102-007-13
Rx only
BACITRACIN OPHTHALMIC
OINTMENT USP, 500 units/g
STERILE
3 x 1 g tubes
Sterile Ophthalmic Ointment
Weighing 1 gram each
USUAL DOSAGE: Apply 1 to 3 times daily.
See package insert for full prescribing information.
EACH GRAM CONTAINS: 500 units of Bacitracin per gram, White Petrolatum, Mineral Oil.
Keep tightly closed.
Store at 20°-25° C (68°-77° F) (See USP
Controlled room temperature).
Keep out of reach of children.
PRINCIPAL DISPLAY PANEL
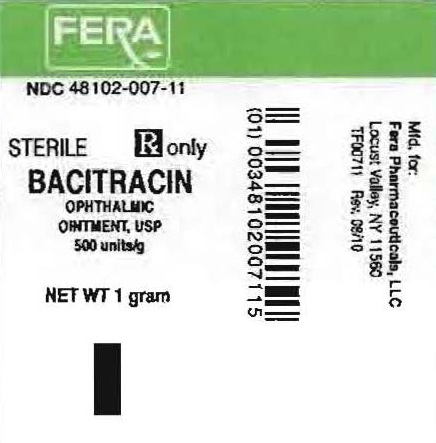
NDC 48102-007-11
STERILE
Rx only
BACITRACIN
OPHTHALMIC
OINTMENT USP
500 units/g
NET WT 1 gm
PRINCIPAL DISPLAY PANEL
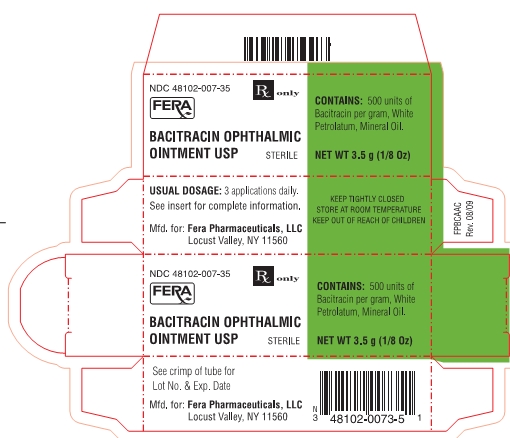
NDC 48102-007-35
Rx only
BACITRACIN OPHTHALMIC
OINTMENT USP
STERILE
CONTAINS: 500 units of Bacitracin per gram, White Petrolatum, Mineral Oil.
NET WT 3.5 g (1/8 Oz)
USUAL DOSAGE: 3 applications daily.
See insert for complete information.
KEEP TIGHTLY CLOSED
STORE AT ROOM TEMPERATURE
KEEP OUT OF REACH OF CHILDREN
See crimp of tube for Lot No. & Exp. Date
PRINCIPAL DISPLAY PANEL

NDC 48102-007-35
BACITRACIN
OPHTHALMIC
OINTMENT USP
STERILE
Rx only
USUAL DOSAGE: 3 applications daily.
See insert for complete information.
WARNING: Keep out of reach of children.
See crimp for Lot No. and Exp. Date
CONTAINS: 500 units of Bacitracin per gram, White Petrolatum, Mineral Oil.
NET WT 3.5 g (1/8 Oz)
KEEP TIGHTLY CLOSED
STORE AT ROOM TEMPERATURE









