NDC Code(s) : 47335-581-40, 47335-581-42
Packager : Sun Pharma Global FZE
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| amifostine amifostine INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
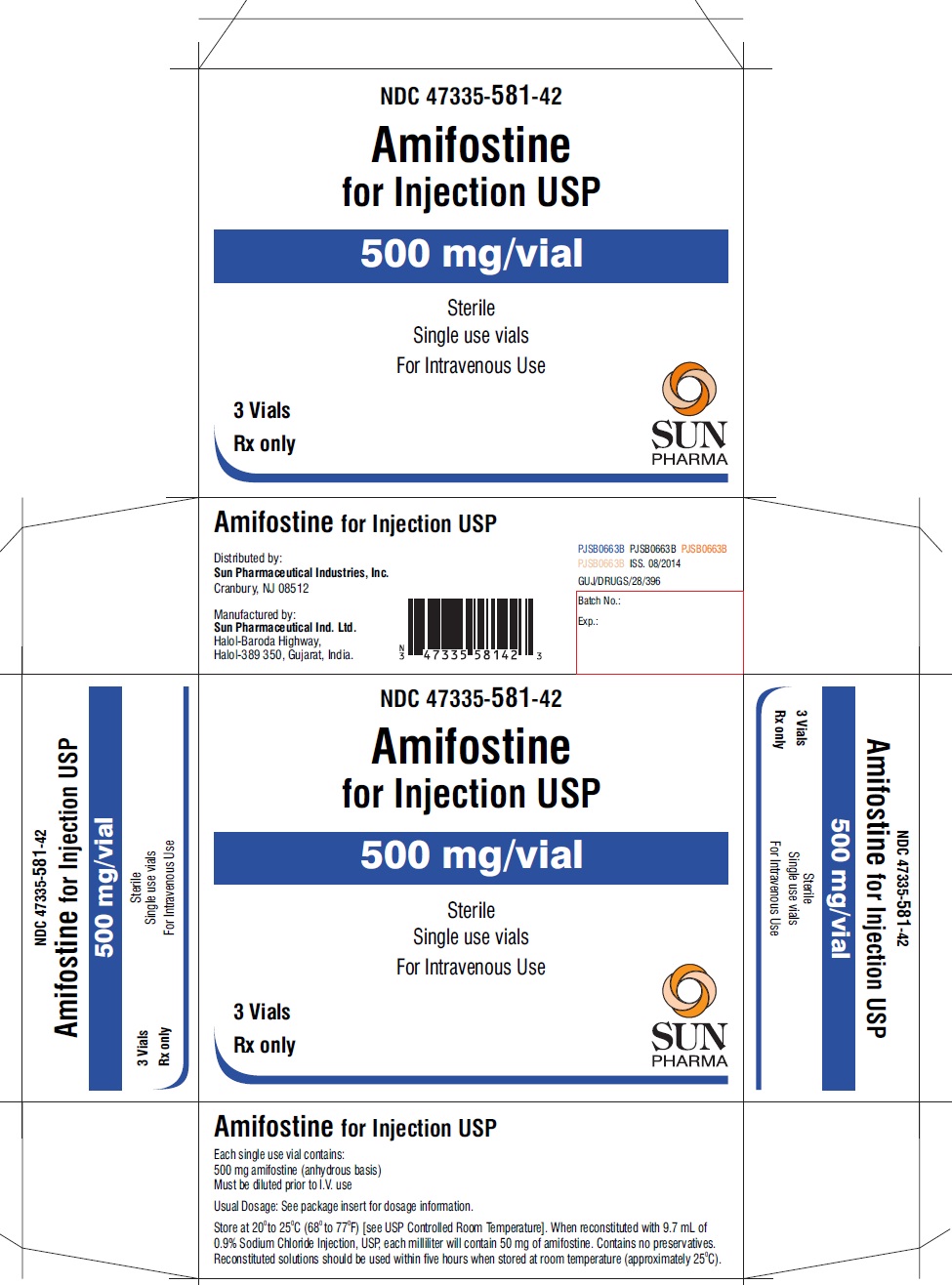
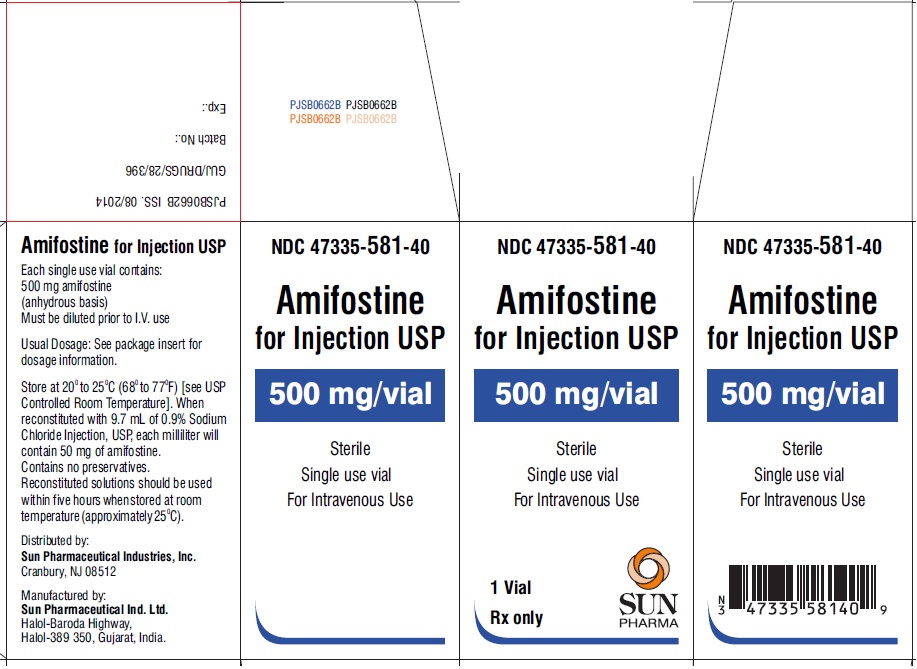
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - vial label
NDC 47335-581-40
Amifostine for Injection USP
500 mg/vial
Sterile
Single use vial
For Intravenous Use
Rx only
PRINCIPAL DISPLAY PANEL - showbox for 1 vial
NDC 47335-581-40
Amifostine for Injection USP
500 mg/vial
Sterile
Single use vials
For Intravenous Use
1 Vial
Rx only
SUN PHARMA
PRINCIPAL DISPLAY PANEL - showbox for 3 vials
NDC 47335-581-42
Amifostine for Injection USP
500 mg/vial
Sterile
Single use vials
For Intravenous Use
3 Vials
Rx only
SUN PHARMA