NDC Code(s) : 42571-382-71, 42571-382-73, 42571-382-27
Packager : Micro Labs Limited
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| DORZOLAMIDE HYDROCHLORIDE AND TIMOLOL MALEATE DORZOLAMIDE HYDROCHLORIDE AND TIMOLOL MALEATE PRESERVATIVE FREE SOLUTION/ DROPS | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| LABELER - Micro Labs Limited(862174955) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Micro Labs Limited | 677600482 | analysis(42571-382), label(42571-382), manufacture(42571-382), pack(42571-382) | |
PRINCIPAL DISPLAY PANEL
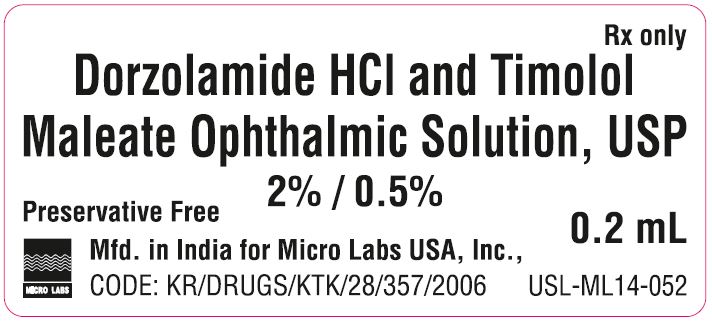
Dorzolamide HCl and Timolol Maleate
Ophthalmic Solution, USP
2% / 0.5%
Preservative Free
For Use in the Eye
Rx only
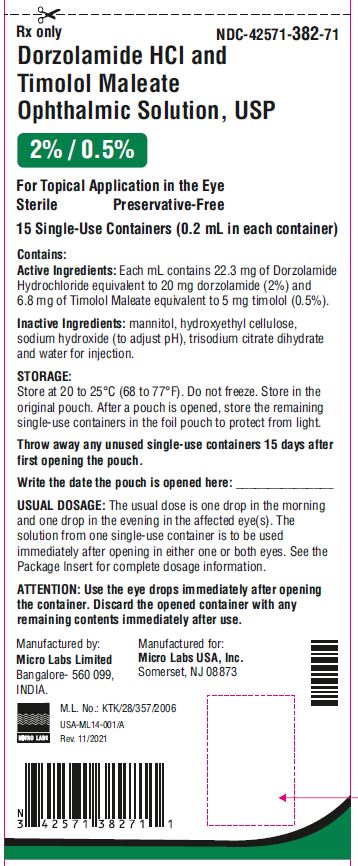
NDC 42571-382-71
Dorzolamide Hydrochloride
and Timolol Maleate
Ophthalmic Solution, USP
2% / 0.5%*
Preservative Free
For Topical Application in the Eye
Rx Only
15 Single-Use Containers
(0.2 mL in each container)
Micro Labs Limited
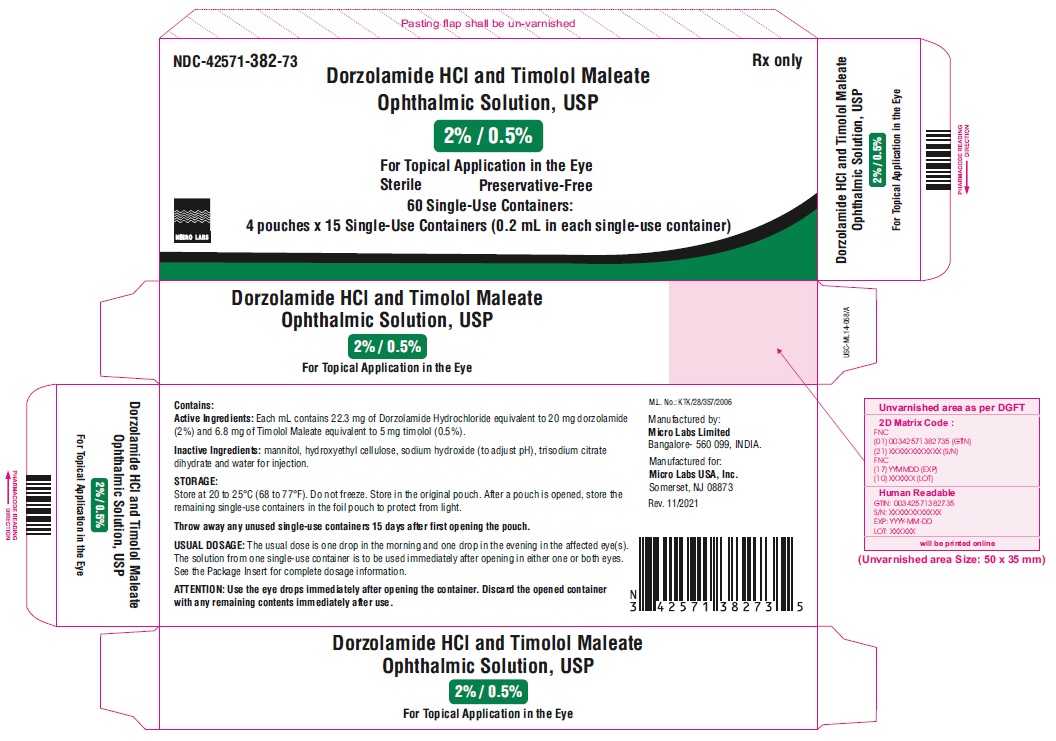
NDC 42571-382-73
Dorzolamide Hydrochloride
and Timolol Maleate
Ophthalmic Solution, USP
2% / 0.5%*
Preservative Free
For Topical Application in the Eye
Rx Only
60 Single-Use Containers:
4 pouches x 15 Single-Use Containers
(0.2 mL in each single-use container)
Micro labs Limited