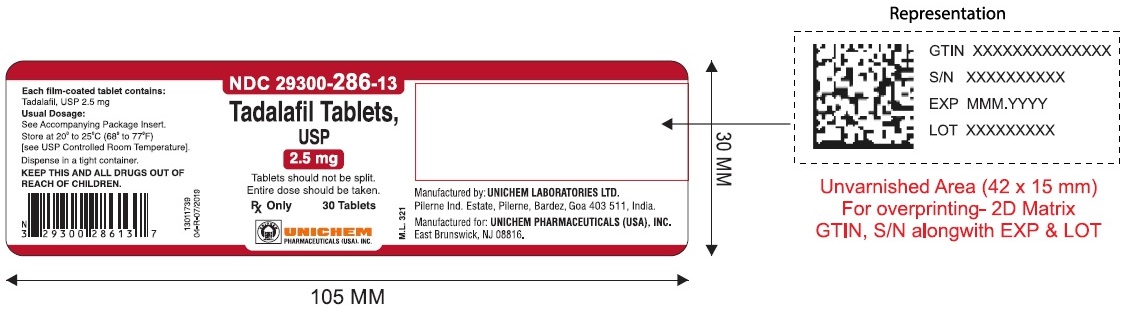
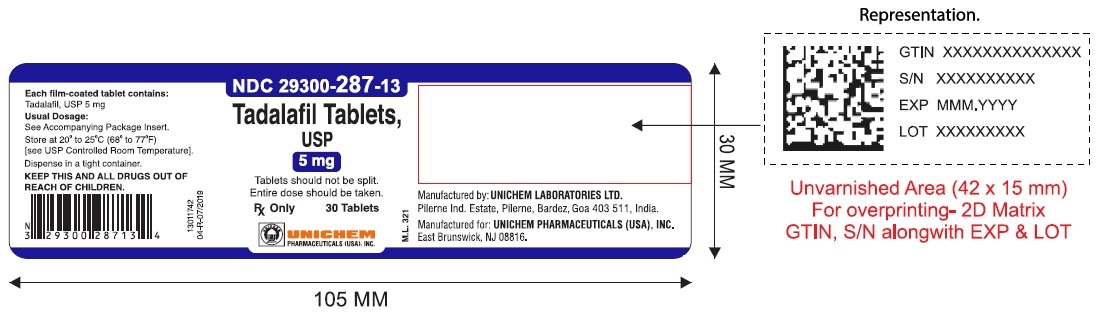
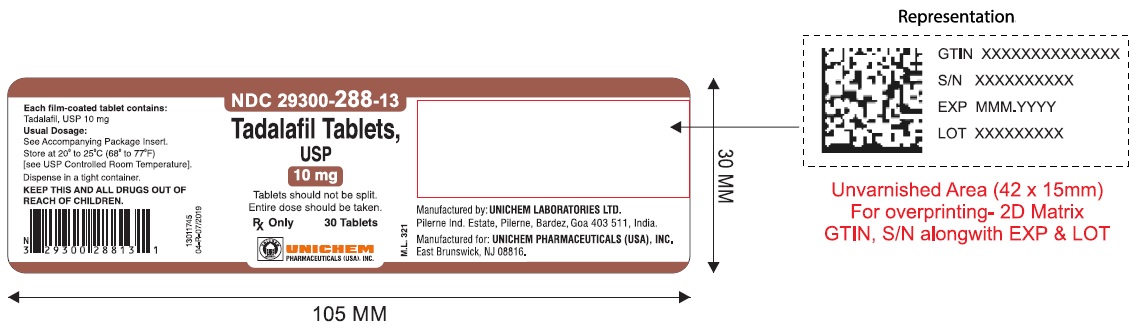
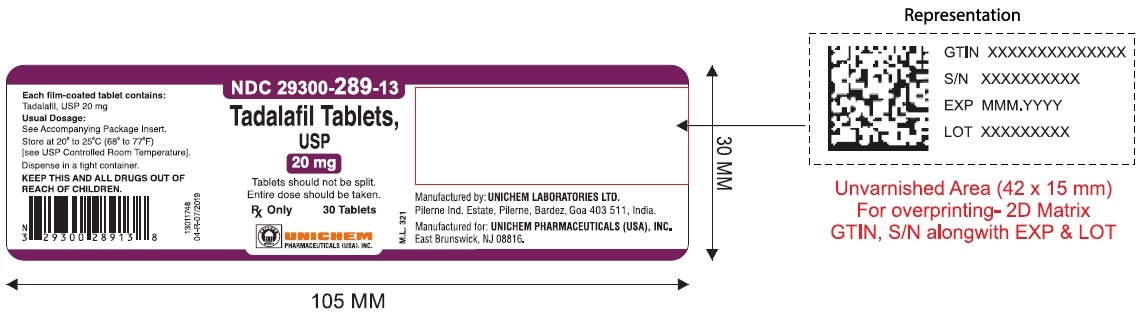
NDC Code(s) : 29300-286-13, 29300-286-01, 29300-286-05, 29300-286-51, 29300-286-82, 29300-287-13, 29300-287-01, 29300-287-05, 29300-287-51, 29300-287-82, 29300-288-13, 29300-288-01, 29300-288-05, 29300-289-13, 29300-289-01, 29300-289-05
Packager : Unichem Pharmaceuticals (USA), Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| tadalafil tadalafil TABLET, FILM COATED | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| tadalafil tadalafil TABLET, FILM COATED | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| tadalafil tadalafil TABLET, FILM COATED | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| tadalafil tadalafil TABLET, FILM COATED | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| LABELER - Unichem Pharmaceuticals (USA), Inc.(181620514) |
PRINCIPAL DISPLAY PANEL