NDC Code(s) : 0548-3012-00, 0548-3013-00, 0548-3015-00, 0548-3011-00
Packager : Amphastar Pharmaceuticals, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Lidocaine HydrochlorideLidocaine Hydrochloride JELLY | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Lidocaine HydrochlorideLidocaine Hydrochloride JELLY | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Lidocaine HydrochlorideLidocaine Hydrochloride JELLY | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Lidocaine HydrochlorideLidocaine Hydrochloride JELLY | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Rx ONLY
NDC 0548-3012-00
STOCK NO. 3012-SP
LIDOCAINE HYDROCHLORIDE JELLY USP, 2%
100 mg (20 mg/mL)
5 mL
STERILE PAK
URO-JET®
FOR TOPICAL USE ONLY / LOCAL ANESTHETIC / USUAL DOSAGE: SEE INSERT
SINGLE USE / NO PRESERVATIVE ADDED / STORE AT CONTROLLED ROOM TEMPERATURE 15° TO 30°C (59° TO 86°F).
EACH mL CONTAINS 20 mg OF LIDOCAINE HYDROCHLORIDE AND SODIUM CARBOXYMETHYLCELLULOSE.
SODIUM HYDROXIDE MAY HAVE BEEN ADDED TO ADJUST pH (pH 6-7).
NOTE: CONTENTS STERILE IN ORIGINAL, INTACT PACKAGE. DO NOT OPEN PACKAGE UNTIL READY TO USE.
SYRINGE ASSEMBLY DIRECTIONS: USE ASEPTIC TECHNIQUE. DO NOT ASSEMBLE UNTIL READY TO USE.
- 1) OPEN STERILE POUCH AND DROP CONTENTS DIRECTLY ONTO STERILE FIELD. REMOVE PROTECTIVE CAPS.
-
2) THREAD VIAL INTO INJECTOR 3 HALF TURNS, OR UNTIL NEEDLE PENETRATES STOPPER. DO NOT PUSH VIAL INTO
INJECTOR; THIS MAY CAUSE MISALIGNMENT.
INTERNATIONAL MEDICATION SYSTEMS, LIMITED SO. EL MONTE, CA 91733, U.S.A.
An Amphastar Pharmaceuticals Company
8730120G 10-03
LOT:
EXP.:

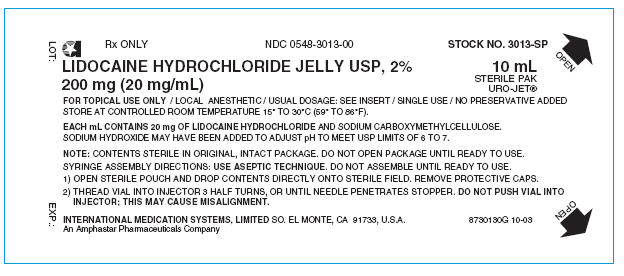
PRINCIPAL DISPLAY PANEL
Rx ONLY
NDC 0548-3013-00
STOCK NO. 3013-SP
LIDOCAINE HYDROCHLORIDE JELLY USP, 2%
200 mg (20 mg/mL)
10 mL
STERILE PAK
URO-JET®
FOR TOPICAL USE ONLY / LOCAL ANESTHETIC / USUAL DOSAGE: SEE INSERT / SINGLE USE / NO PRESERVATIVE ADDED
STORE AT CONTROLLED ROOM TEMPERATURE 15° TO 30°C (59° TO 86°F).
EACH mL CONTAINS 20 mg OF LIDOCAINE HYDROCHLORIDE AND SODIUM CARBOXYMETHYLCELLULOSE.
SODIUM HYDROXIDE MAY HAVE BEEN ADDED TO ADJUST pH TO MEET USP LIMITS OF 6 TO 7.
NOTE: CONTENTS STERILE IN ORIGINAL, INTACT PACKAGE. DO NOT OPEN PACKAGE UNTIL READY TO USE.
SYRINGE ASSEMBLY DIRECTIONS: USE ASEPTIC TECHNIQUE. DO NOT ASSEMBLE UNTIL READY TO USE.
- 1) OPEN STERILE POUCH AND DROP CONTENTS DIRECTLY ONTO STERILE FIELD. REMOVE PROTECTIVE CAPS.
-
2) THREAD VIAL INTO INJECTOR 3 HALF TURNS, OR UNTIL NEEDLE PENETRATES STOPPER. DO NOT PUSH VIAL INTO
INJECTOR; THIS MAY CAUSE MISALIGNMENT.
INTERNATIONAL MEDICATION SYSTEMS, LIMITED SO. EL MONTE, CA 91733, U.S.A.
An Amphastar Pharmaceuticals Company
8730130G 10-03
LOT:
EXP.:
PRINCIPAL DISPLAY PANEL
Rx ONLY
NDC 0548-3015-00
STOCK NO. 3015-SP
LIDOCAINE HYDROCHLORIDE JELLY USP, 2%
400 mg (20 mg/mL)
20 mL
STERILE PAK
URO-JET®
FOR TOPICAL USE ONLY / LOCAL ANESTHETIC / USUAL DOSAGE: SEE INSERT / SINGLE USE / NO PRESERVATIVE ADDED
STORE AT CONTROLLED ROOM TEMPERATURE 15° TO 30°C (59° TO 86°F).
EACH mL CONTAINS 20 mg OF LIDOCAINE HYDROCHLORIDE AND SODIUM CARBOXYMETHYLCELLULOSE.
SODIUM HYDROXIDE MAY HAVE BEEN ADDED TO ADJUST pH TO MEET USP LIMITS OF 6 TO 7.
NOTE: CONTENTS STERILE IN ORIGINAL, INTACT PACKAGE. DO NOT OPEN PACKAGE UNTIL READY TO USE.
SYRINGE ASSEMBLY DIRECTIONS: USE ASEPTIC TECHNIQUE. DO NOT ASSEMBLE UNTIL READY TO USE.
- 1) OPEN STERILE POUCH AND DROP CONTENTS DIRECTLY ONTO STERILE FIELD. REMOVE PROTECTIVE CAPS.
-
2) THREAD VIAL INTO INJECTOR 3 HALF TURNS, OR UNTIL NEEDLE PENETRATES STOPPER. DO NOT PUSH VIAL INTO
INJECTOR; THIS MAY CAUSE MISALIGNMENT.
INTERNATIONAL MEDICATION SYSTEMS, LIMITED SO. EL MONTE, CA 91733, U.S.A.
An Amphastar Pharmaceuticals Company
8730150G 10-03
LOT:
EXP.:

PRINCIPAL DISPLAY PANEL
NDC 0548-3011-00
STOCK NO. 3011-SP
LIDOCAINE HYDROCHLORIDE JELLY USP, 2%
100 mg (20 mg/mL)
REDUCED SIZE TIP
5 mL
STERILE PAK?URO-JET®
AC?(ANATOMICALLY
CONSTRICTED)
FOR TOPICAL USE ONLY / LOCAL ANESTHETIC
USUAL DOSAGE: SEE INSERT / SINGLE USE / NO PRESERVATIVE ADDED?STORE AT CONTROLLED ROOM
TEMPERATURE 15° TO 30°C (59° TO 86°F).
EACH mL CONTAINS 20 mg OF LIDOCAINE HYDROCHLORIDE AND SODIUM CARBOXYMETHYLCELLULOSE
AS A VISCOSITY-INCREASING AGENT. SODIUM HYDROXIDE MAY HAVE BEEN ADDED TO ADJUST pH TO MEET
USP LIMITS OF 6 TO 7.
NOTE: CONTENTS STERILE IN ORIGINAL, INTACT PACKAGE. DO NOT OPEN PACKAGE UNTIL READY TO USE.
Rx ONLY
INTERNATIONAL MEDICATION SYSTEMS, LIMITED SO. EL MONTE, CA 91733 USA 8730110B 4-98
LOT:
EXP.:










