NDC Code(s) : 0338-3410-50, 0338-3410-24, 0338-3612-50, 0338-3612-24, 0338-3814-50, 0338-3814-24
Packager : Baxter Healthcare Corporation
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Clindamycin phosphateClindamycin phosphate INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Clindamycin phosphateClindamycin phosphate INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Clindamycin phosphateClindamycin phosphate INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Baxter Healthcare Corporation(005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Baxter Healthcare Corporation | 194684502 | ANALYSIS(0338-3410, 0338-3612, 0338-3814), LABEL(0338-3814, 0338-3612, 0338-3814), MANUFACTURE(0338-3410, 0338-3612, 0338-3814), PACK(0338-3410, 0338-3612, 0338-3814), STERILIZE(0338-3410, 0338-3612, 0338-3814) | |
PRINCIPAL DISPLAY PANEL
 Container Label
Container Label
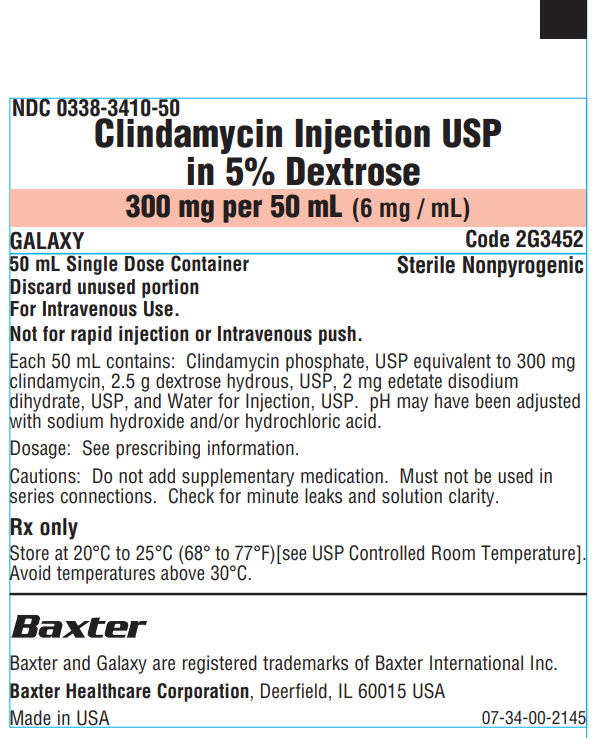
NDC 0338-3410-50
Clindamycin Injection USP
In 5% Dextrose
300 mg per 50 mL (6 mg / mL)
GALAXY
Code 2G3452
50 mL Single Dose Container
Discard unused portion
For Intravenous Use.
Sterile Nonpyrogenic
Not for rapid injection or Intravenous push.
Each 50 mL contains: Clindamycin phosphate, USP equivalent to 300 mg
clindamycin, 2.5 g dextrose hydrous, USP, 2 mg edetate disodium
dihydrate, USP, and Water for Injection, USP. pH may have been adjusted
with sodium hydroxide and/or hydrochloric acid.
Dosage: See prescribing information.
Cautions: Do not add supplementary medication. Must not be used in
series connections. Check for minute leaks and solution clarity.
Rx only
Store at 20°C to 25°C (68° to 77°F)[see USP Controlled Room Temperature].
Avoid temperatures above 30°C.
Baxter Logo
Baxter and Galaxy are registered trademarks of Baxter International Inc.
Baxter Healthcare Corporation, Deerfield, IL 60015 USA
Made in USA
07-34-00-2145
BAR CODE POSITION ONLY
303383410504
 Carton Label
Carton Label
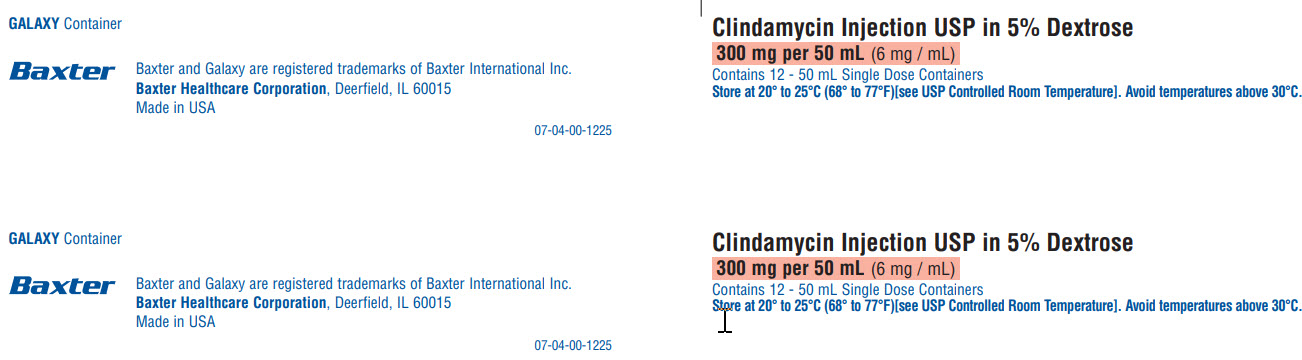
GALAXY Container
Baxter Logo
Baxter and Galaxy are registered trademarks of Baxter International Inc.
Baxter Healthcare Corporation, Deerfield, IL 60015
Made in USA
07-04-00-1225
Clindamycin Injection USP in 5% Dextrose
300 mg per 50 mL (6 mg / mL)
Contains 12 - 50 mL Single Dose Containers
Store at 20° to 25°C (68° to 77°F)[see USP Controlled Room Temperature]. Avoid temperatures above 30°C.
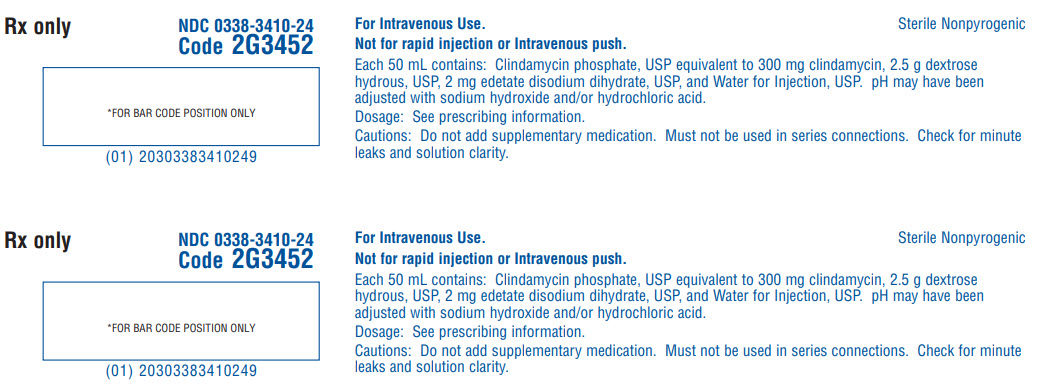
Rx only
NDC 0338-3410-24
Code 2G3452
*FOR BAR CODE POSITION ONLY
(01) 20303383410249
For Intravenous Use.
Not for rapid injection or Intravenous push.
Sterile Nonpyrogenic
Each 50 mL contains: Clindamycin phosphate, USP equivalent to 300 mg clindamycin, 2.5 g dextrose
hydrous, USP, 2 mg edetate disodium dihydrate, USP, and Water for Injection, USP. pH may have been
adjusted with sodium hydroxide and/or hydrochloric acid.
Dosage: See prescribing information.
Cautions: Do not add supplementary medication. Must not be used in series connections. Check for minute leaks and solution clarity.

 Container Label
Container Label
NDC 0338-3612-50
Clindamycin Injection USP
In 5% Dextrose
600 mg per 50 mL
(12 mg / mL)
GALAXY
Code 2G3453
50 mL Single Dose Container
Discard unused portion
For Intravenous Use.
Not for rapid injection or Intravenous push.
Sterile Nonpyrogenic
Each 50 mL contains: Clindamycin phosphate, USP equivalent to 600 mg
clindamycin, 2.5 g dextrose hydrous, USP, 2 mg edetate disodium
dihydrate, USP, and Water for Injection, USP. pH may have been adjusted
with sodium hydroxide and/or hydrochloric acid.
Dosage: See prescribing information.
Cautions: Do not add supplementary medication. Must not be used in
series connections. Check for minute leaks and solution clarity.
Rx only
Store at 20°C to 25°C (68° to 77°F)[see USP Controlled Room Temperature].
Avoid temperatures above 30°C.
Baxter Logo
Baxter and Galaxy are registered trademarks of Baxter International Inc.
Baxter Healthcare Corporation, Deerfield, IL 60015 USA
Made in USA
07-34-00-2146
BAR CODE POSITION ONLY
303383612502
 Carton Label
Carton Label
GALAXY Container
Baxter Logo
Baxter and Galaxy are registered trademarks of Baxter International Inc.
Baxter Healthcare Corporation, Deerfield, IL 60015
Made in USA
07-04-00-1226
Clindamycin Injection USP in 5% Dextrose
600 mg per 50 mL (12 mg / mL)
Contains 12 - 50 mL Single Dose Containers
Store at 20° to 25°C (68° to 77°F)[see USP Controlled Room Temperature]. Avoid temperatures above 30°C.
Rx only
NDC 0338-3612-24
Code 2G3453
*FOR BAR CODE POSITION ONLY
(01) 20303383612247
For Intravenous Use.
Not for rapid injection or Intravenous push.
Sterile Nonpyrogenic
Each 50 mL contains: Clindamycin phosphate, USP equivalent to 600 mg clindamycin, 2.5 g dextrose
hydrous, USP, 2 mg edetate disodium dihydrate, USP, and Water for Injection, USP. pH may have been
adjusted with sodium hydroxide and/or hydrochloric acid.
Dosage: See prescribing information.
Cautions: Do not add supplementary medication. Must not be used in series connections. Check for minute leaks and solution clarity.
 Container Label
Container Label
NDC 0338-3814-50
Clindamycin Injection USP
In 5% Dextrose
900 mg per 50 mL (18 mg / mL)
GALAXY
Code 2G3454
50 mL Single Dose Container
Discard unused portion
For Intravenous Use.
Sterile Nonpyrogenic
Not for rapid injection or Intravenous push.
Each 50 mL contains: Clindamycin phosphate, USP equivalent to 900 mg
clindamycin, 2.5 g dextrose hydrous, USP, 2 mg edetate disodium
dihydrate, USP, and Water for Injection, USP. pH may have been adjusted
with sodium hydroxide and/or hydrochloric acid.
Dosage: See prescribing information.
Cautions: Do not add supplementary medication. Must not be used in
series connections. Check for minute leaks and solution clarity.
Rx only
Store at 20°C to 25°C (68° to 77°F)[see USP Controlled Room Temperature].
Avoid temperatures above 30°C.
Baxter Logo
Baxter and Galaxy are registered trademarks of Baxter International Inc.
Baxter Healthcare Corporation, Deerfield, IL 60015 USA
Made in USA
07-34-00-2147
BAR CODE POSITION ONLY
303383814500
 Carton Label
Carton Label
GALAXY Container
Baxter Logo
Baxter and Galaxy are registered trademarks of Baxter International Inc.
Baxter Healthcare Corporation, Deerfield, IL 60015
Made in USA
07-04-00-1227
Clindamycin Injection USP in 5% Dextrose
900 mg per 50 mL (18 mg / mL)
Contains 12 - 50 mL Single Dose Containers
Store at 20° to 25°C (68° to 77°F)[see USP Controlled Room Temperature]. Avoid temperatures above 30°C.
Rx only
NDC 0338-3814-24
Code 2G3454
*FOR BAR CODE POSITION ONLY
(01) 20303383814245
For Intravenous Use.
Not for rapid injection or Intravenous push.
Sterile Nonpyrogenic
Each 50 mL contains: Clindamycin phosphate, USP equivalent to 900 mg clindamycin, 2.5 g dextrose
hydrous, USP, 2 mg edetate disodium dihydrate, USP, and Water for Injection, USP. pH may have been
adjusted with sodium hydroxide and/or hydrochloric acid.
Dosage: See prescribing information.
Cautions: Do not add supplementary medication. Must not be used in series connections. Check for minute leaks and solution clarity.









