NDC Code(s) : 0264-7510-00, 0264-7510-10, 0264-7510-20, 0264-7520-00, 0264-7520-10, 0264-7520-20
Packager : B. Braun Medical Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| DEXTROSEDEXTROSE SOLUTION | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| DEXTROSEDEXTROSE SOLUTION | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| LABELER - B. Braun Medical Inc.(002397347) |
PRINCIPAL DISPLAY PANEL
5% Dextrose
Injection USP
REF L5100
NDC 0264-7510-00
DIN 01924281
HK 22598
1000 mL
EXCEL® CONTAINER
Each 100 mL contains: Hydrous Dextrose USP 5 g;
Water for Injection USP qs
pH: 4.4 (3.5-6.5); Calc. Osmolarity: 250 mOsmol/liter
Sterile, nonpyrogenic. Single dose container. Do not use in
series connection. For intravenous use only. Use only if
solution is clear and container and seals are intact.
WARNINGS: ELECTROLYTE-FREE DEXTROSE SOLUTIONS
SHOULD NOT BE GIVEN CONJOINTLY WITH BLOOD BECAUSE
AGGLOMERATION MAY OCCUR. Some additives may be
incompatible. Consult with pharmacist. When introducing
additives, use aseptic techniques. Mix thoroughly.
Do not store.
Recommended Storage: Room temperature (25°C). Avoid
excessive heat. Protect from freezing. See Package Insert.
Do not remove overwrap until ready for use. After removing the
overwrap, check for minute leaks by squeezing container firmly. If
leaks are found, discard solution as sterility may be impaired.
Not made with natural rubber latex, PVC or DEHP.
Rx only
EXCEL is a registered trademark of B. Braun Medical Inc.
7
OTHER
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
www.bbraun.com
In Canada, distributed by:
B. Braun of Canada, Ltd.
Scarborough, Ontario M1H 2W4
Y94-003-242
LD-111-3
EXP
LOT

PRINCIPAL DISPLAY PANEL
5% Dextrose Injection USP
REF L5101
NDC 0264-7510-10
DIN 01924281
HK 22598
500 mL
EXCEL® CONTAINER
Each 100 mL contains: Hydrous Dextrose USP 5 g;
Water for Injection USP qs
pH: 4.4 (3.5-6.5); Calc. Osmolarity: 250 mOsmol/liter
Sterile, nonpyrogenic. Single dose container. Do not use in series
connection. For intravenous use only. Use only if solution is clear and
container and seals are intact.
WARNINGS: ELECTROLYTE-FREE DEXTROSE SOLUTIONS SHOULD NOT BE
GIVEN CONJOINTLY WITH BLOOD BECAUSE AGGLOMERATION MAY OCCUR.
Some additives may be incompatible. Consult with pharmacist. When
introducing additives, use aseptic techniques. Mix thoroughly. Do not store.
Recommended Storage: Room temperature (25°C). Avoid excessive heat.
Protect from freezing. See Package Insert.
Do not remove overwrap until ready for use. After removing the overwrap, check
for minute leaks by squeezing container firmly. If leaks are found, discard
solution as sterility may be impaired.
Not made with natural rubber latex, PVC or DEHP.
Rx only
7
OTHER
EXCEL is a registered trademark of B. Braun Medical Inc.
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
www.bbraun.com
In Canada, distributed by:
B. Braun of Canada, Ltd.
Scarborough, Ontario M1H 2W4
Y94-003-240
LD-118-3
EXP
LOT
 Y94-003-240
Y94-003-240
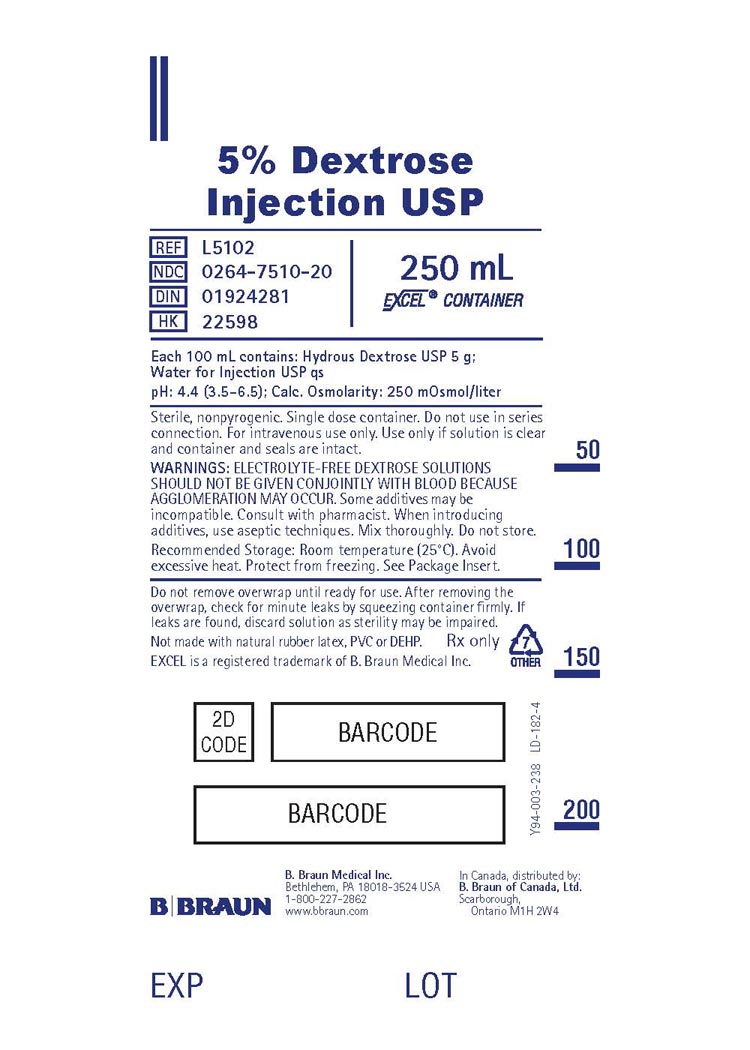
PRINCIPAL DISPLAY PANEL
5% Dextrose
Injection USP
REF L5102
NDC 0264-7510-20
DIN 01924281
HK 22598
250 mL
EXCEL® CONTAINER
Each 100 mL contains: Hydrous Dextrose USP 5 g;
Water for Injection USP qs
pH: 4.4 (3.5-6.5); Calc. Osmolarity: 250 mOsmol/liter
Sterile, nonpyrogenic. Single dose container. Do not use in series
connection. For intravenous use only. Use only if solution is clear
and container and seals are intact.
WARNINGS: ELECTROLYTE-FREE DEXTROSE SOLUTIONS
SHOULD NOT BE GIVEN CONJOINTLY WITH BLOOD BECAUSE
AGGLOMERATION MAY OCCUR. Some additives may be
incompatible. Consult with pharmacist. When introducing
additives, use aseptic techniques. Mix thoroughly. Do not store.
Recommended Storage: Room temperature (25°C). Avoid
excessive heat. Protect from freezing. See Package Insert.
Do not remove overwrap until ready for use. After removing the
overwrap, check for minute leaks by squeezing container firmly. If
leaks are found, discard solution as sterility may be impaired.
Not made with natural rubber latex, PVC or DEHP.
Rx only
EXCEL is a registered trademark of B. Braun Medical Inc.
7
OTHER
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
www.bbraun.com
In Canada, distributed by:
B. Braun of Canada, Ltd.
Scarborough, Ontario M1H 2W4
Y94-003-238
LD-182-4
EXP
LOT
 Y94-003-238
Y94-003-238
PRINCIPAL DISPLAY PANEL
10% Dextrose
Injection USP
REF L5200
NDC 0264-7520-00
DIN 01924427
HK 22599
1000 mL
EXCEL® CONTAINER
Each 100 mL contains: Hydrous Dextrose USP 10 g;
Water for Injection USP qs
pH: 4.4 (3.5-6.5); Calc. Osmolarity: 505 mOsmol/liter,
hypertonic
Sterile, nonpyrogenic. Single dose container. Do not use in
series connection. For intravenous use only. Use only if
solution is clear and container and seals are intact.
WARNINGS: ELECTROLYTE-FREE DEXTROSE SOLUTIONS
SHOULD NOT BE GIVEN CONJOINTLY WITH BLOOD BECAUSE
AGGLOMERATION MAY OCCUR. Some additives may be
incompatible. Consult with pharmacist. When introducing
additives, use aseptic techniques. Mix thoroughly.
Do not store.
Recommended Storage: Room temperature (25°C). Avoid
excessive heat. Protect from freezing. See Package Insert.
Do not remove overwrap until ready for use. After removing the
overwrap, check for minute leaks by squeezing container firmly. If
leaks are found, discard solution as sterility may be impaired.
Not made with natural rubber latex, PVC or DEHP.
Rx only
EXCEL is a registered trademark of B. Braun Medical Inc.
7
OTHER
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
www.bbraun.com
In Canada, distributed by:
B. Braun of Canada, Ltd.
Scarborough, Ontario M1H 2W4
Y94-003-241
LD-181-3
EXP
LOT

PRINCIPAL DISPLAY PANEL
10% Dextrose Injection USP
REF L5201
NDC 0264-7520-10
DIN 01924427
HK 22599
500 mL
EXCEL® CONTAINER
Each 100 mL contains: Hydrous Dextrose USP 10 g;
Water for Injection USP qs
pH: 4.4 (3.5-6.5); Calc. Osmolarity: 505 mOsmol/liter, hypertonic
Sterile, nonpyrogenic. Single dose container. Do not use in series
connection. For intravenous use only. Use only if solution is clear and
container and seals are intact.
WARNINGS: ELECTROLYTE-FREE DEXTROSE SOLUTIONS SHOULD NOT BE
GIVEN CONJOINTLY WITH BLOOD BECAUSE AGGLOMERATION MAY OCCUR.
Some additives may be incompatible. Consult with pharmacist. When
introducing additives, use aseptic techniques. Mix thoroughly. Do not store.
Recommended Storage: Room temperature (25°C). Avoid excessive heat.
Protect from freezing. See Package Insert.
Do not remove overwrap until ready for use. After removing the overwrap, check
for minute leaks by squeezing container firmly. If leaks are found, discard
solution as sterility may be impaired.
Not made with natural rubber latex, PVC or DEHP.
Rx only
EXCEL is a registered trademark of B. Braun Medical Inc.
7
OTHER
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
www.bbraun.com
In Canada, distributed by:
B. Braun of Canada, Ltd.
Scarborough, Ontario M1H 2W4
Y94-003-239
LD-180-3
EXP
LOT
 Y94-003-239
Y94-003-239
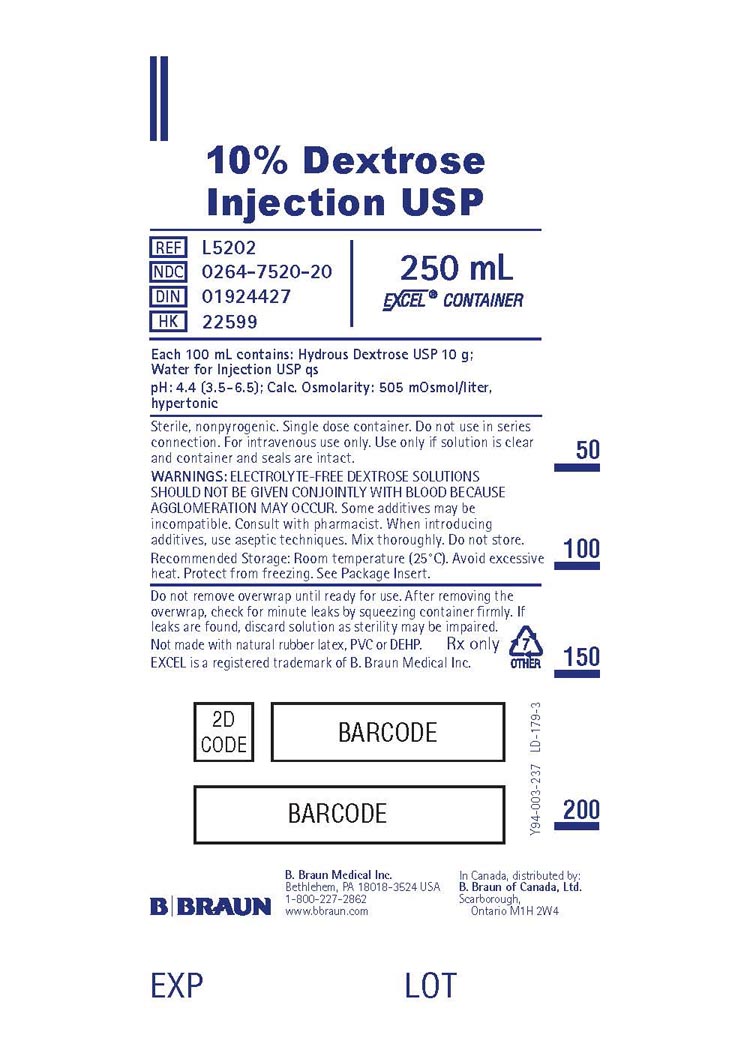
PRINCIPAL DISPLAY PANEL
10% Dextrose
Injection USP
REF L5202
NDC 0264-7520-20
DIN 01924427
HK 22599
250 mL
EXCEL® CONTAINER
Each 100 mL contains: Hydrous Dextrose USP 10 g;
Water for Injection USP qs
pH: 4.4 (3.5-6.5); Calc. Osmolarity: 505 mOsmol/liter,
hypertonic
Sterile, nonpyrogenic. Single dose container. Do not use in series
connection. For intravenous use only. Use only if solution is clear
and container and seals are intact.
WARNINGS: ELECTROLYTE-FREE DEXTROSE SOLUTIONS
SHOULD NOT BE GIVEN CONJOINTLY WITH BLOOD BECAUSE
AGGLOMERATION MAY OCCUR. Some additives may be
incompatible. Consult with pharmacist. When introducing
additives, use aseptic techniques. Mix thoroughly. Do not store.
Recommended Storage: Room temperature (25°C). Avoid excessive
heat. Protect from freezing. See Package Insert.
Do not remove overwrap until ready for use. After removing
the overwrap, check for minute leaks by squeezing container firmly. If
leaks are found, discard solution as sterility may be impaired.
Not made with natural rubber latex, PVC or DEHP.
Rx only
EXCEL is a registered trademark of B. Braun Medical Inc.
7
OTHER
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
www.bbraun.com
In Canada, distributed by:
B. Braun of Canada, Ltd.
Scarborough, Ontario M1H 2W4
Y94-003-237
LD-179-3
EXP
LOT
 Y94-003-237
Y94-003-237









