NDC Code(s) : 0228-2127-10, 0228-2127-50, 0228-2128-10, 0228-2128-50, 0228-2129-10
Packager : Actavis Pharma, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Clonidine HydrochlorideClonidine Hydrochloride TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Clonidine HydrochlorideClonidine Hydrochloride TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Clonidine HydrochlorideClonidine Hydrochloride TABLET | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| LABELER - Actavis Pharma, Inc.(119723554) |
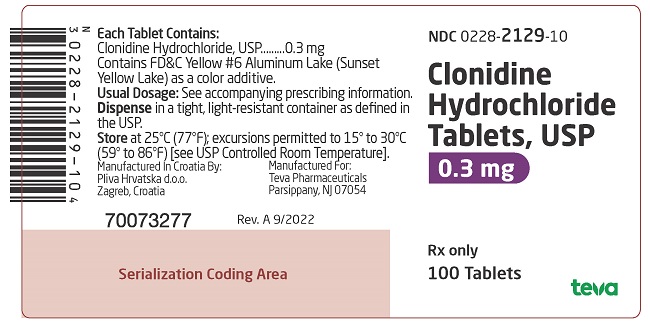
PRINCIPAL DISPLAY PANEL
NDC 0228-2127-10
Clonidine Hydrochloride Tablets, USP
0.1 mg
Rx only
100 Tablets
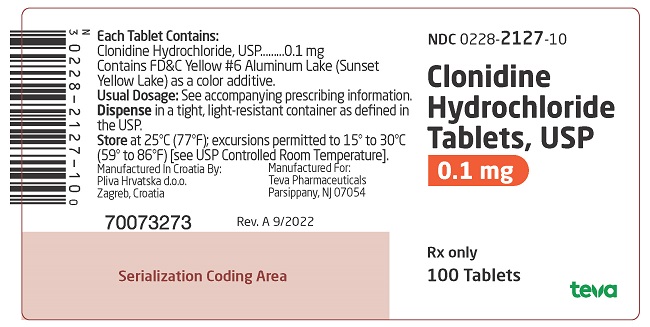
PRINCIPAL DISPLAY PANEL
NDC 0228-2128-10
Clonidine Hydrochloride Tablets, USP
0.2 mg
Rx only
100 Tablets
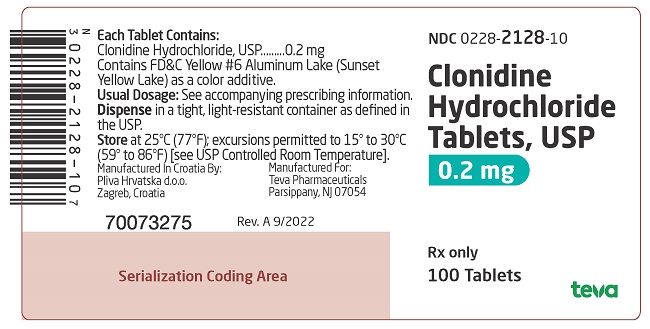
PRINCIPAL DISPLAY PANEL
NDC 0228-2129-10
Clonidine Hydrochloride Tablets, USP
0.3 mg
Rx only
100 Tablets