NDC Code(s) : 0206-2404-01, 0206-2404-02, 0206-2405-01, 0206-2405-02, 0206-2408-01, 0206-2408-02, 0206-2416-01, 0206-2409-01, 0206-2409-02, 0206-2411-01, 0206-2411-02, 0206-2413-01, 0206-2413-02
Packager : Wyeth Pharmaceuticals Inc., a subsidiary of Pfizer Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Zosynpiperacillin sodium and tazobactam sodium INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Zosynpiperacillin sodium and tazobactam sodium INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Zosynpiperacillin sodium and tazobactam sodium INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Zosynpiperacillin sodium and tazobactam sodium INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Zosyn PIPERACILLIN SODIUM and TAZOBACTAM SODIUM INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Zosyn PIPERACILLIN SODIUM and TAZOBACTAM SODIUM INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Zosyn PIPERACILLIN SODIUM and TAZOBACTAM SODIUM INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
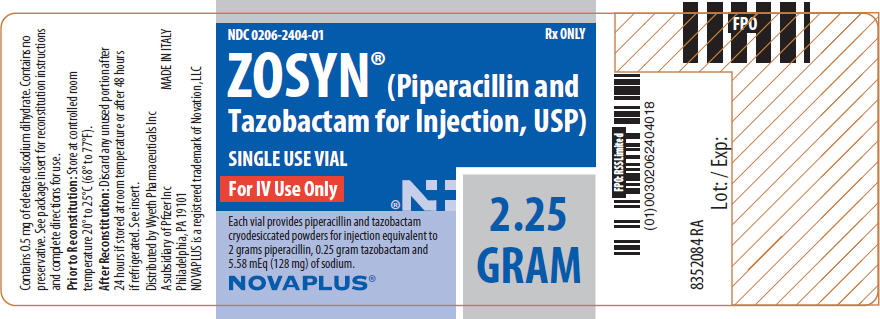
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 2.25 GRAM - LABEL
NDC 0206-2404-01
ZOSYN®
(Piperacillin and Tazobactam for Injection, USP)
2.25 GRAM
SINGLE USE VIAL
Each vial provides piperacillin and tazobactam cryodesiccated powders for injection equivalent to 2 grams piperacillin, 0.25 gram tazobactam and 5.58 mEq (128 mg) of sodium.
For IV Use Only
Rx only
NOVAPLUS®
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 2.25 GRAM - CARTON
NDC 0206-2404-02
ZOSYN®
(Piperacillin and Tazobactam for Injection, USP)
2.25 GRAM
10 x 2.25 GRAM SINGLE USE VIALS
For IV Use Only
Rx only
NOVAPLUS®

PRINCIPAL DISPLAY PANEL
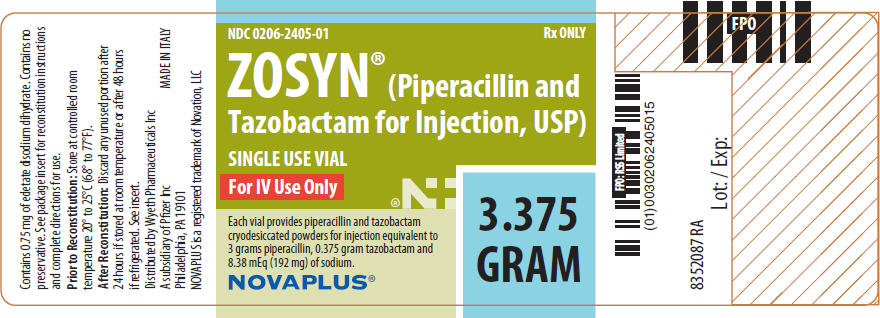
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 3.375 GRAM - LABEL
NDC 0206-2405-01
ZOSYN®
(Piperacillin and Tazobactam for Injection, USP)
3.375 GRAM
SINGLE USE VIAL
Each vial provides piperacillin and tazobactam cryodesiccated powders for injection equivalent to 3 grams piperacillin, 0.375 gram tazobactam and 8.38 mEq (192 mg) of sodium.
For IV Use Only
Rx only
NOVAPLUS®
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 3.375 GRAM - CARTON
NDC 0206-2405-02
ZOSYN®
(Piperacillin and Tazobactam for Injection, USP)
3.375 GRAM
10 x 3.375 GRAM SINGLE USE VIALS
For IV Use Only
Rx only
NOVAPLUS®

PRINCIPAL DISPLAY PANEL
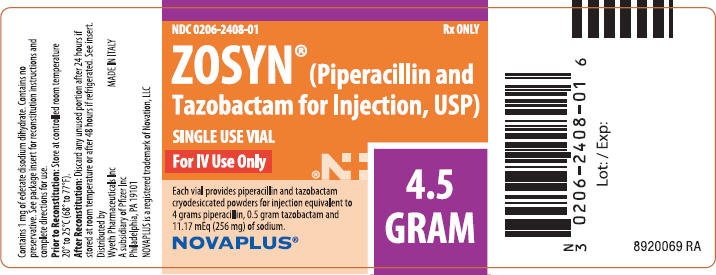
PRINCIPAL DISPLAY PANEL - 4.5 GRAM Vial Label
NDC 0206-2408-01
Rx ONLY
ZOSYN® (Piperacillin and
Tazobactam for Injection, USP)
SINGLE USE VIAL
For IV Use Only
Each vial provides piperacillin and tazobactam
cryodesiccated powders for injection equivalent to
4 grams piperacillin, 0.5 gram tazobactam and
11.17 mEq (256 mg) of sodium.
NOVAPLUS®
4.5
GRAM
PRINCIPAL DISPLAY PANEL
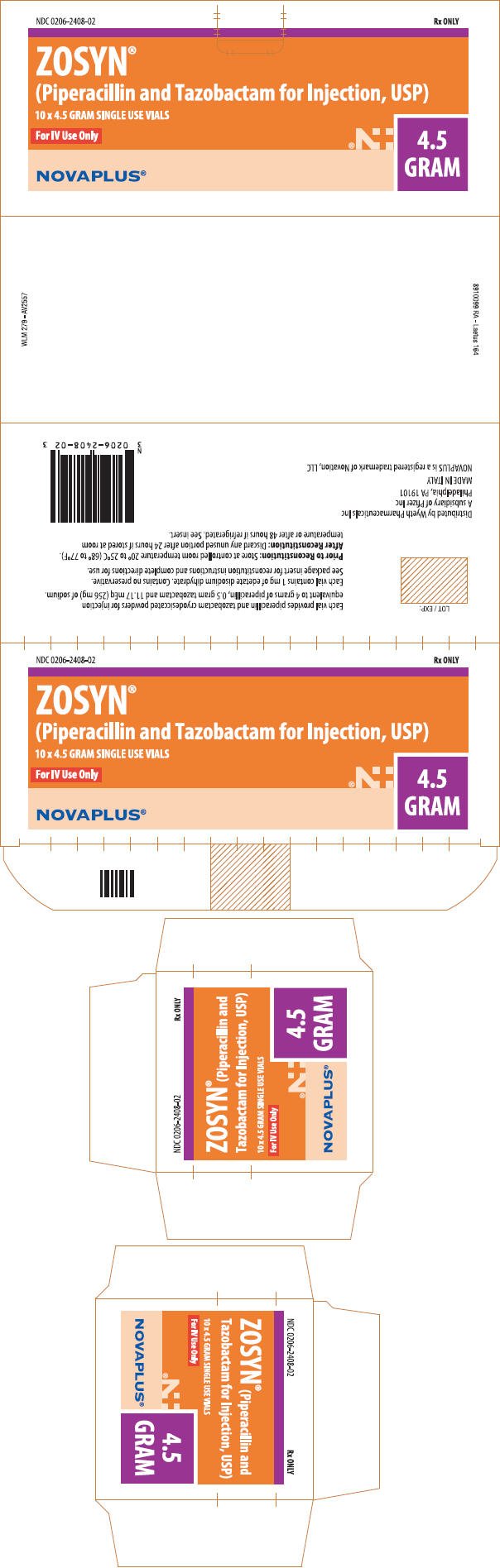
PRINCIPAL DISPLAY PANEL - 4.5 GRAM Vial Carton
NDC 0206-2408-02
Rx ONLY
ZOSYN®
(Piperacillin and Tazobactam for Injection, USP)
10 x 4.5 GRAM SINGLE USE VIALS
For IV Use Only
4.5
GRAM
NOVAPLUS®
PRINCIPAL DISPLAY PANEL
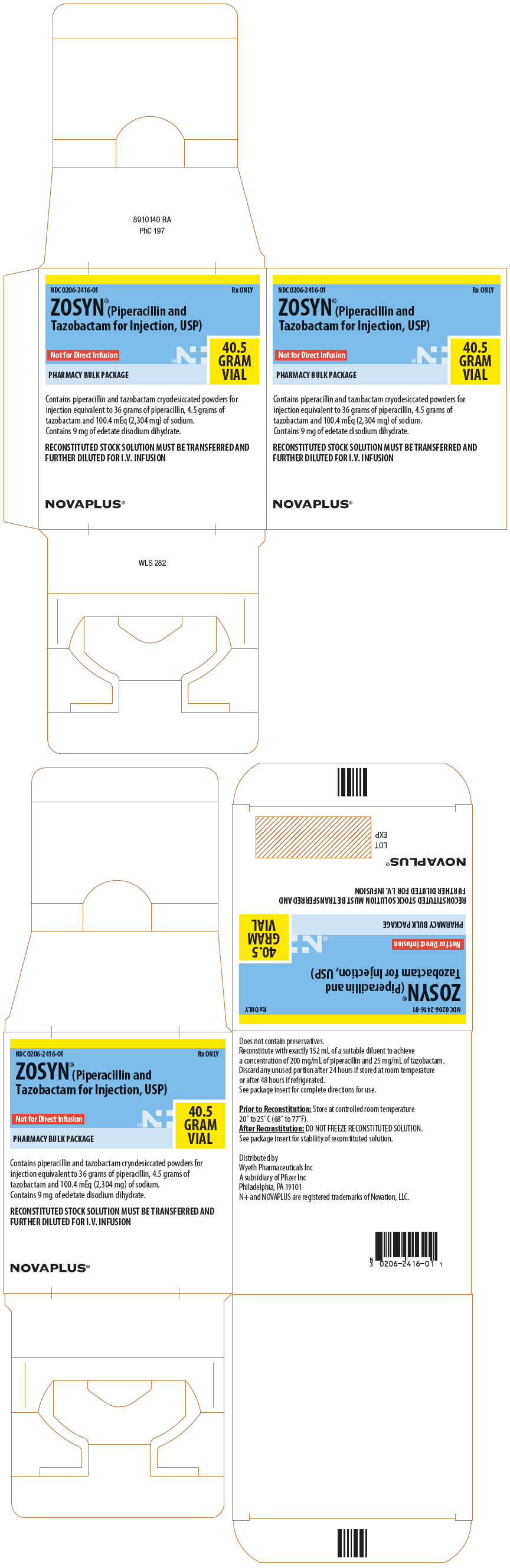
NOVAPLUS®
PHARMACY BULK PACKAGE
NDC 0206-2416-01
Rx ONLY
ZOSYN®(Piperacillin and
Tazobactam for Injection, USP)
Not for Direct Infusion
RECONSTITUTED STOCK SOLUTION MUST
BE TRANSFERRED AND FURTHER
DILUTED FOR I.V. INFUSION
40.5
GRAM
VIAL

PRINCIPAL DISPLAY PANEL
NDC 0206-2416-01
Rx ONLY
ZOSYN®(Piperacillin and
Tazobactam for Injection, USP)
Not for Direct Infusion
PHARMACY BULK PACKAGE
40.5
GRAM
VIAL
Contains piperacillin and tazobactam cryodesiccated powders for
injection equivalent to 36 grams of piperacillin, 4.5 grams of
tazobactam and 100.4 mEq (2,304 mg) of sodium.
Contains 9 mg of edetate disodium dihydrate.
RECONSTITUTED STOCK SOLUTION MUST BE TRANSFERRED AND
FURTHER DILUTED FOR I.V. INFUSION
NOVAPLUS®
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 2.25 G SINGLE DOSE CONTAINER
NDC 0206-2409-01
ZOSYN®
(Piperacillin and Tazobactam Injection)
2.25 g
NOVAPLUS®
GALAXY
Single Dose
Container
Code 2G3585
50 mL Iso-osmotic
Sterile Nonpyrogenic
Rx ONLY

PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 2.25 G - LABEL UNIT
NDC 0206-2409-02
ZOSYN®
(Piperacillin and Tazobactam Injection)
2.25 g
NOVAPLUS®
Code 2G3585
12 - 50 mL Single Dose Containers
Iso-osmotic
Store at or below -20°C/-4°F.
Do not refreeze
Rx ONLY

PRINCIPAL DISPLAY PANEL
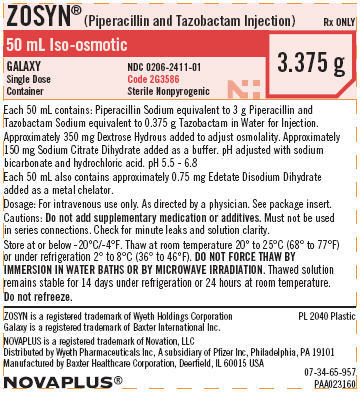
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 3.375 G - SINGLE DOSE CONTAINER
NDC 0206-2411-01
ZOSYN®
(Piperacillin and Tazobactam Injection)
3.375 g
NOVAPLUS®
GALAXY
Single Dose
Container
50 mL Iso-osmotic
Code 2G3586
Sterile Nonpyrogenic
Rx ONLY
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 3.375 G - LABEL UNIT
NDC 0206-2411-02
ZOSYN®
(Piperacillin and Tazobactam Injection)
3.375 g
NOVAPLUS®
Code 2G3586
12 - 50 mL Single Dose Containers
Iso-osmotic
Store at or below -20°C/-4°F.
Do not refreeze
Rx ONLY

PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 4.5 G - SINGLE DOSE CONTAINER
NDC 0206-2413-01
ZOSYN®
(Piperacillin and Tazobactam Injection)
4.5 g
NOVAPLUS®
GALAXY
Single Dose
Container
100 mL Iso-osmotic
Code 2G3587
Sterile Nonpyrogenic
Rx ONLY

PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 4.5 G - LABEL UNIT
NDC 0206-2413-02
ZOSYN®
(Piperacillin and Tazobactam Injection)
4.5 g
NOVAPLUS®
Code 2G3587
6 - 100 mL Single Dose Containers
Iso-osmotic
Store at or below -20°C/-4°F.
Do not refreeze
Rx ONLY

NOVAPLUS is a registered trademark of Vizient, Inc.
LAB-0713-8.0









