NDC Code(s) : 0116-1061-08, 0116-1061-16, 0116-1061-32, 0116-1061-01, 0116-1061-40, 0116-1061-18
Packager : Xttrium Laboratories, Inc.
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| ANTISEPTIC SKIN CLEANSERCHLORHEXIDINE GLUCONATE SOLUTION | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| LABELER - Xttrium Laboratories, Inc.(007470579) |
| REGISTRANT - Xttrium Laboratories, Inc.(007470579) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Xttrium Laboratories, Inc. | 007470579 | manufacture(0116-1061) | |
PRINCIPAL DISPLAY PANEL
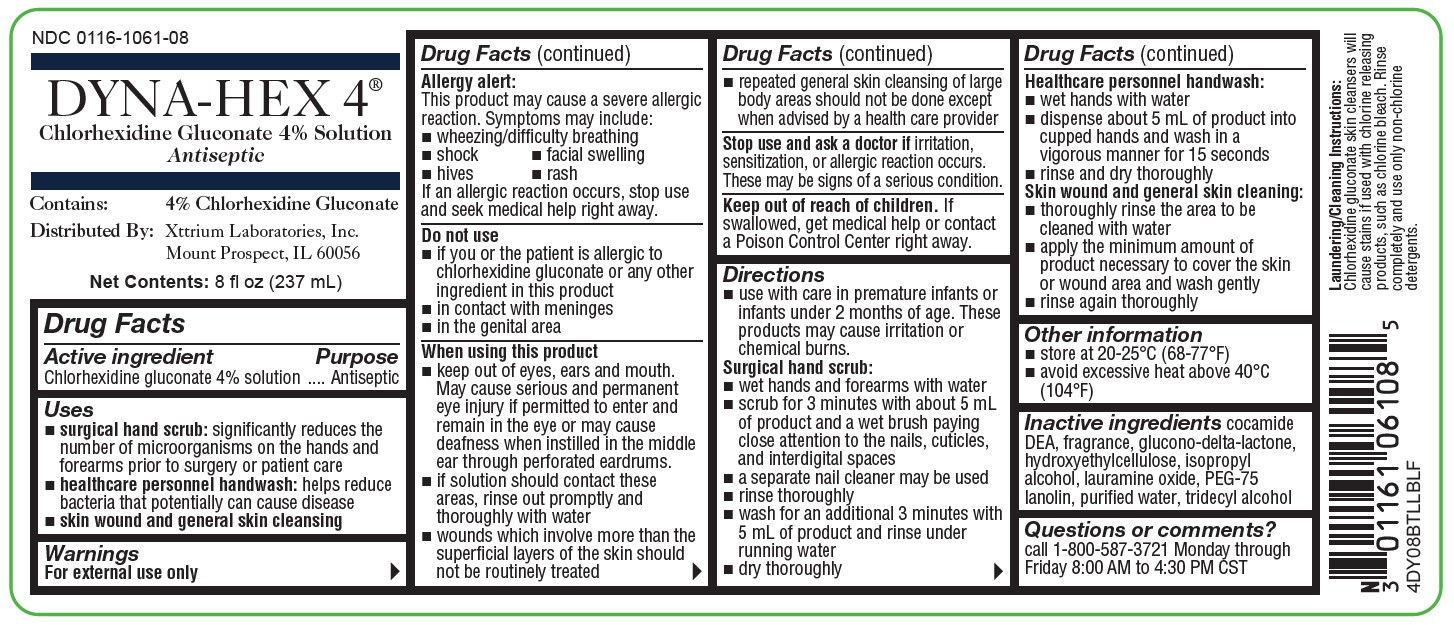
NDC 0116-1061-08
DYNA-HEX 4 ®
(Chlorhexidine Gluconate 4% Solution)
Antiseptic
Contains: 4% Chlorhexidine Gluconate
Distributed by: Xttrium Laboratories, Inc., Mount Prospect, IL 60056
FOR EXTERNAL USE ONLY
Net Contents: 8 fl oz (237 mL)
4DY08BTLLBLF
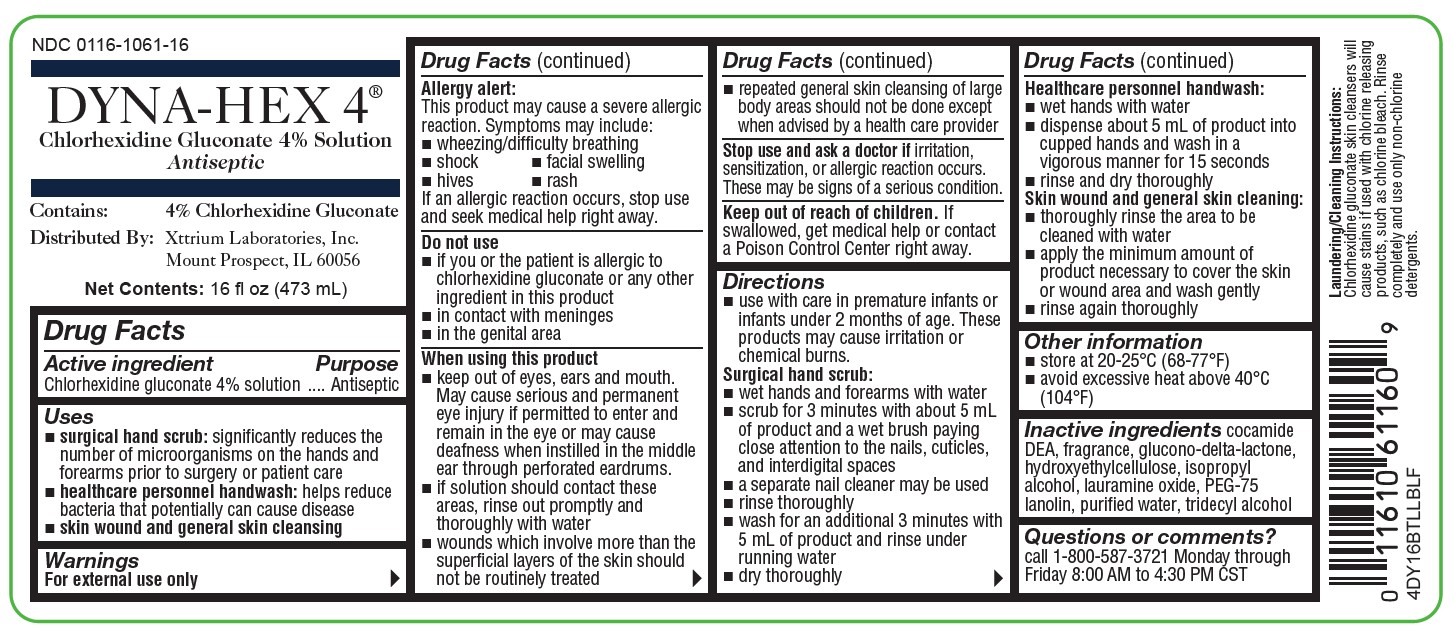
NDC 0116-1061-16
DYNA-HEX 4 ®
(Chlorhexidine Gluconate 4% Solution)
Antiseptic
Contains: 4% Chlorhexidine Gluconate
Distributed by: Xttrium Laboratories, Inc., Mount Prospect, IL 60056
FOR EXTERNAL USE ONLY
Net Contents: 16 fl oz (473 mL)
4DY16BTLLBLF
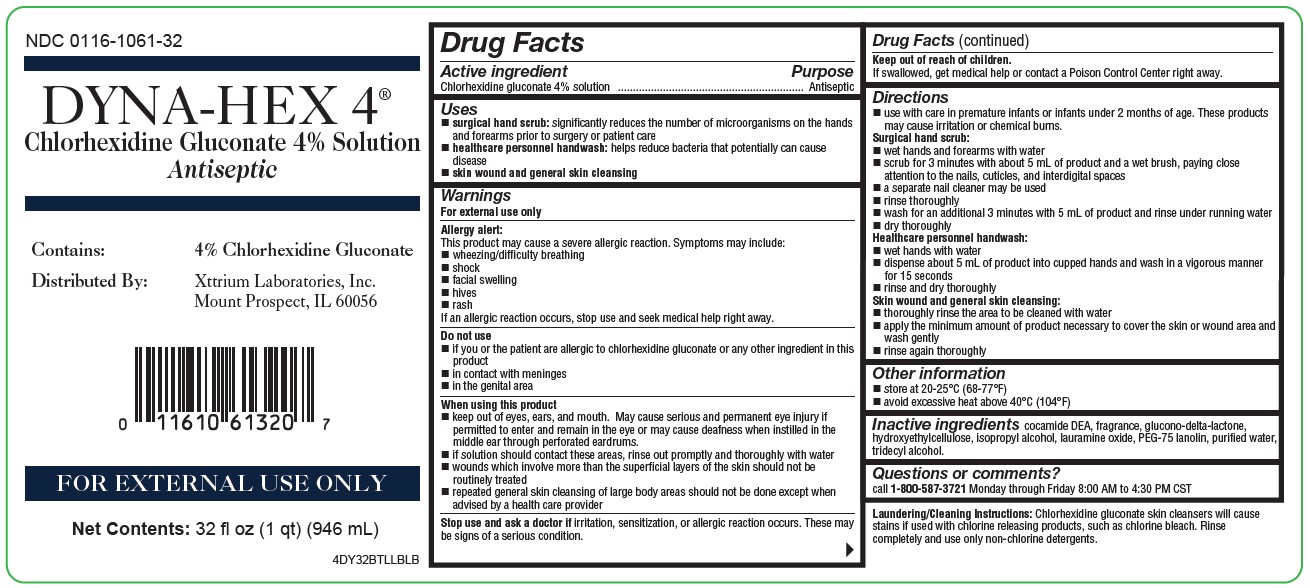
NDC 0116-1061-32
DYNA-HEX 4 ®
(Chlorhexidine Gluconate 4% Solution)
Antiseptic
Contains: 4% Chlorhexidine Gluconate
Distributed by: Xttrium Laboratories, Inc., Mount Prospect, IL 60056
FOR EXTERNAL USE ONLY
Net Contents: 32 fl oz (946 mL)
4DY32BTLLBLB
NDC 0116-1061-01
DYNA-HEX 4 ®
(Chlorhexidine Gluconate 4% Solution)
Antiseptic
Contains: 4% Chlorhexidine Gluconate
Distributed by: Xttrium Laboratories, Inc., Mount Prospect, IL 60056
FOR EXTERNAL USE ONLY
Net Contents: 128 fl oz (1 gal)
4DYN1GBTLLBLC

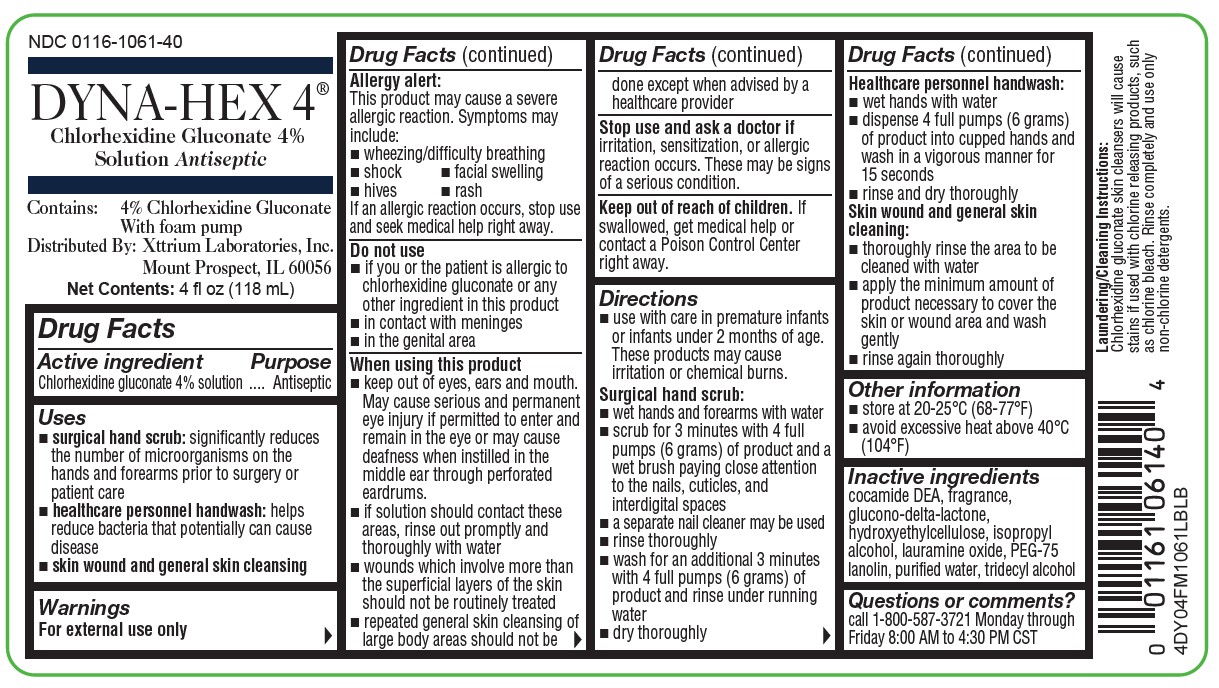
NDC 0116-1061-40
DYNA-HEX 4®
Chlorhexidine Gluconate 4% Solution
Antiseptic
Contains:
4% Chlorhexidine Gluconate With foam pump
Distributed By:
Xttrium Laboratories, Inc.
Mount Prospect, IL 60056
FOR EXTERNAL USE ONLY
Net Contents: 4 fl oz (118mL)
4DY04FM1061LBLB
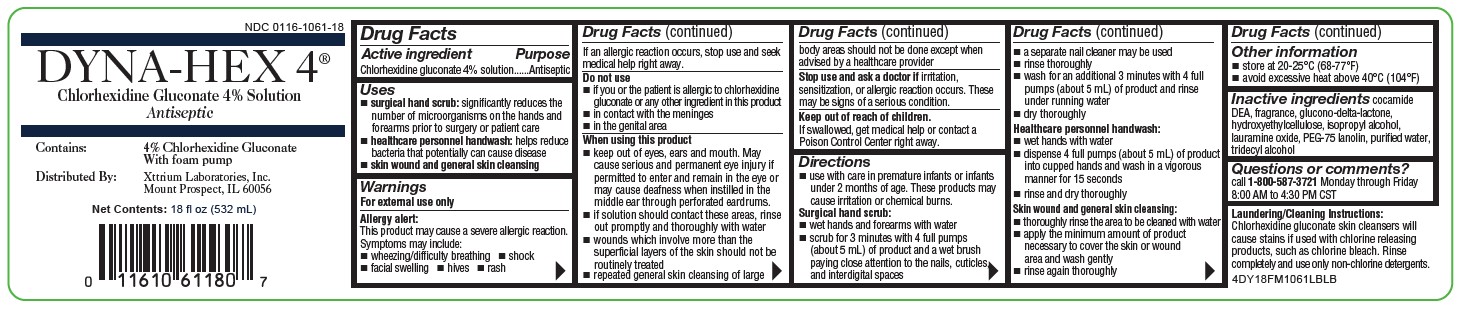
NDC 0116-1061-18
DYNA-HEX 4®
Chlorhexidine Gluconate 4% Solution
Antiseptic
Contains:
4% Chlorhexidine Gluconate
Distributed By:
Xttrium Laboratories, Inc.
Mount Prospect, IL 60056
FOR EXTERNAL USE ONLY
Net Contents: 18 fl oz (532mL)
4DY18FM1061LBLB