NDC Code(s) : 0116-0175-16
Packager : Xttrium Laboratories, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Chlorhexidine GluconateChlorhexidine Gluconate RINSE | ||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| LABELER - Xttrium Laboratories, Inc.(007470579) |
| REGISTRANT - Xttrium Laboratories, Inc.(007470579) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Xttrium Laboratories, Inc. | 007470579 | manufacture(0116-0175) | |
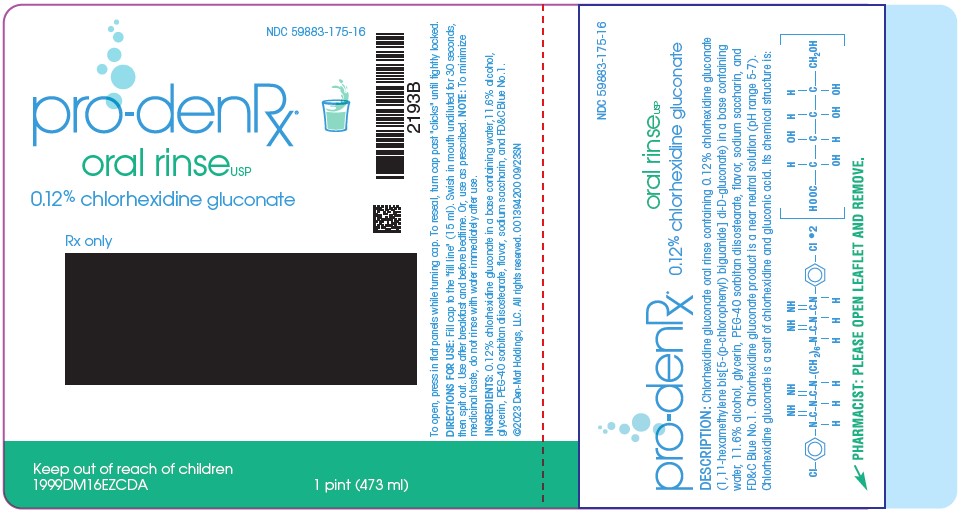
PRINCIPAL DISPLAY PANEL
NDC 59883-175-16
pro-denRx
oral rinse USP
0.12% chlorhexidine gluconate
Rx Only
Keep out of reach of children
To open, press in flat panels while turning cap. Ro reseal, turn cap past "clicks" until tightly locked.
DIRECTIONS FOR USE: Fill cap to the "fill line" (15 mL). Swish in mouth undiluted for 30 seconds, then spit out. Use after breakfast and before bedtime. Or, use as prescribed. NOTE: To minimize medicinal taste, do not rinse with water immediately after use.
INGREDIENTS: 0.12% chlorhexidine gluconate in a base containing water, 11.6% alcohol, glycerin, PEG-40 sorbitan diisostearate, flavor, sodium saccharin, and FD&C Blue No. 1.
1 Pint (473 mL)
1999DM16BLDLA