NDC Code(s) : 0093-3016-30, 0093-3016-65, 0093-3017-30, 0093-3017-65, 0093-3017-56, 0093-3018-56, 0093-3019-56
Packager : Teva Pharmaceuticals USA, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| TadalafilTadalafil TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| TadalafilTadalafil TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| TadalafilTadalafil TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| TadalafilTadalafil TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Teva Pharmaceuticals USA, Inc.(001627975) |
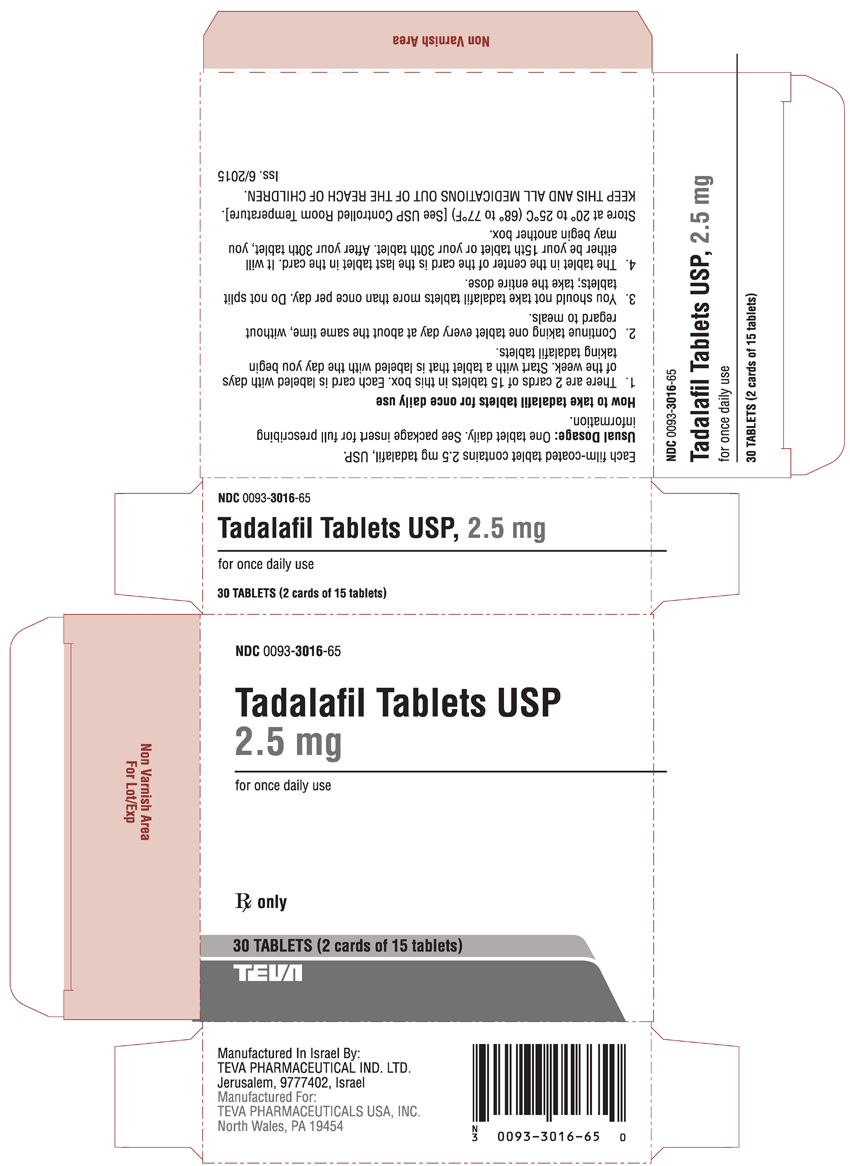
PRINCIPAL DISPLAY PANEL
NDC 0093-3016-56
Tadalafil Tablets USP
2.5 mg
for once daily use
Rx only
30 TABLETS (2 cards of 15 tablets)
TEVA
PRINCIPAL DISPLAY PANEL
NDC 0093-3017-56
Tadalafil
Tablets USP
5 mg
Tablets should not be split.
Entire dose should be taken.
Rx only
30 TABLETS
TEVA

PRINCIPAL DISPLAY PANEL
NDC 0093-3018-56
Tadalafil
Tablets USP
10 mg
Tablets should not be split.
Entire dose should be taken.
Rx only
30 TABLETS
TEVA

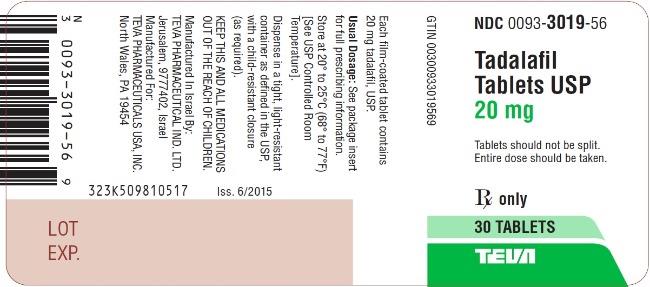
PRINCIPAL DISPLAY PANEL
NDC 0093-3019-56
Tadalafil
Tablets USP
20 mg
Tablets should not be split.
Entire dose should be taken.
Rx only
30 TABLETS
TEVA