NDC Code(s) : 0075-0624-31, 0075-0620-41, 0075-0621-61, 0075-0622-81, 0075-0623-01, 0075-0626-04, 0075-2912-02, 0075-2915-02
Packager : sanofi-aventis U.S. LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Lovenoxenoxaparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Lovenoxenoxaparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Lovenoxenoxaparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Lovenoxenoxaparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Lovenoxenoxaparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Lovenoxenoxaparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Lovenoxenoxaparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Lovenoxenoxaparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 30mg/0.3mL Carton
NDC 0075-0624-31
Lovenox®
(enoxaparin sodium injection)
30mg/0.3mL
Rx ONLY
SINGLE DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.3mL Syringes
NOVAPLUS™

PRINCIPAL DISPLAY PANEL
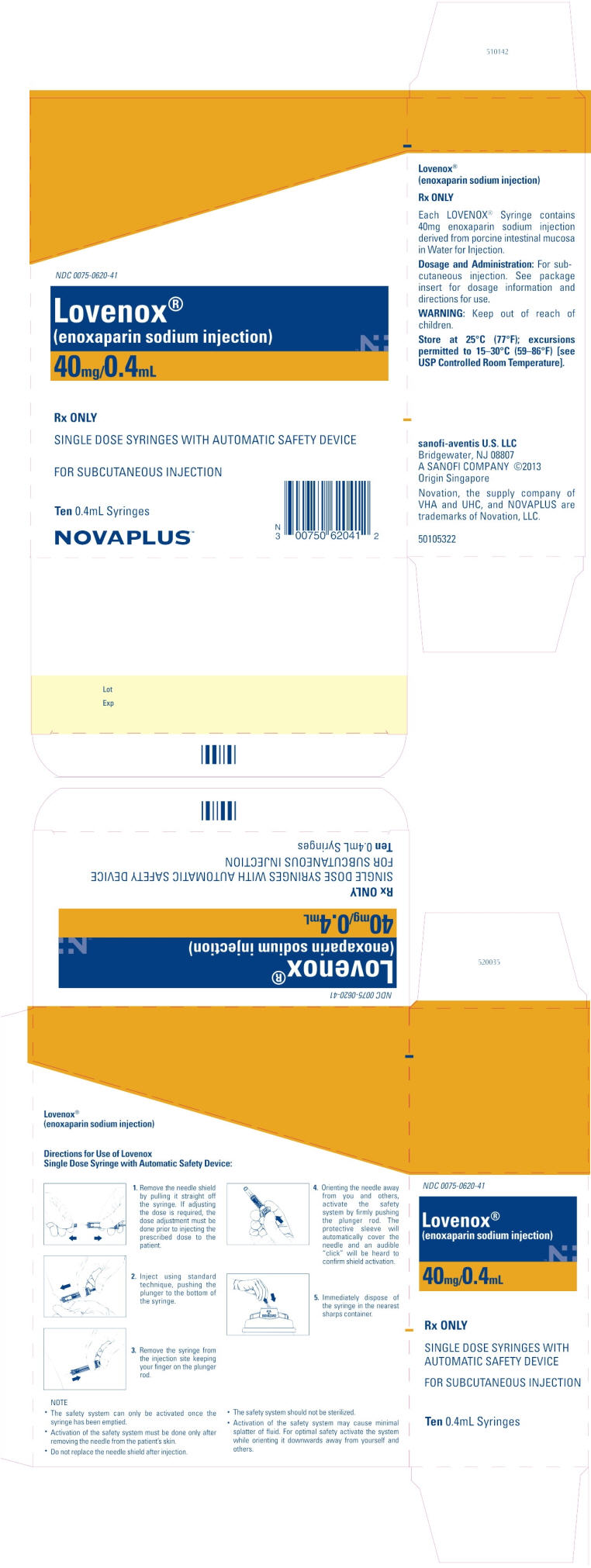
PRINCIPAL DISPLAY PANEL - 40mg/0.4mL Carton
NDC 0075-0620-41
Lovenox®
(enoxaparin sodium injection)
40mg/0.4mL
Rx ONLY
SINGLE DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.4mL Syringes
NOVAPLUS™
PRINCIPAL DISPLAY PANEL
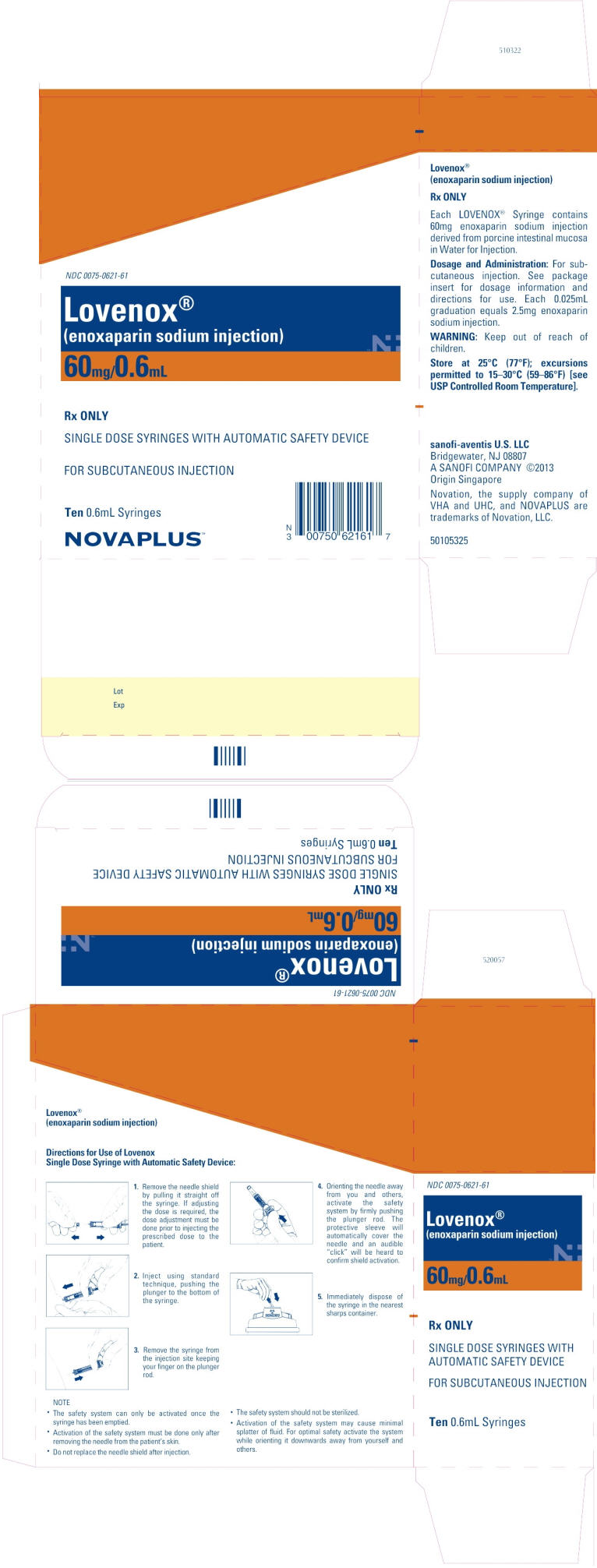
PRINCIPAL DISPLAY PANEL - 60mg/0.6mL Carton
NDC 0075-0621-61
Lovenox®
(enoxaparin sodium injection)
60mg/0.6mL
Rx ONLY
SINGLE DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.6mL Syringes
NOVAPLUS™
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 80mg/0.8mL Carton
NDC 0075-0622-81
Lovenox®
(enoxaparin sodium injection)
80mg/0.8mL
Rx ONLY
SINGLE DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.8mL Syringes
NOVAPLUS™

PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 100mg/1mL Carton
NDC 0075-0623-01
Lovenox®
(enoxaparin sodium injection)
100mg/1mL
Rx ONLY
SINGLE DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 1mL Syringes
NOVAPLUS™

PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 300mg/3mL Carton
NDC 0075-0626-04
Lovenox®
(enoxaparin sodium injection)
300mg/3mL
(100mg/1mL)
Rx ONLY
For Subcutaneous or
Intravenous Injection
Multiple Dose Vial
One 3mL Vial
NOVAPLUS™

PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 120mg/0.8mL Carton
NDC 0075-2912-02
Lovenox®
(enoxaparin sodium injection)
120mg/0.8mL
Rx ONLY
SINGLE DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.8mL Syringes
NOVAPLUS™

PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 150mg/1mL Carton
NDC 0075-2915-02
Lovenox®
(enoxaparin sodium injection)
150mg/1mL
Rx ONLY
SINGLE DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 1mL Syringes
NOVAPLUS™









