NDC Code(s) : 0069-3241-15, 0069-3241-22, 0069-3342-15, 0069-3342-22, 0069-4395-19, 0069-4396-27
Packager : Pfizer Labs, Division of Pfizer Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Ciprofloxacin Ciprofloxacin INJECTION, SOLUTION, CONCENTRATE | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Ciprofloxacin Ciprofloxacin INJECTION, SOLUTION, CONCENTRATE | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Ciprofloxacin Ciprofloxacin INJECTION, SOLUTION | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Ciprofloxacin Ciprofloxacin INJECTION, SOLUTION | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
PRINCIPAL DISPLAY PANEL
TO OPEN - TEAR AT NOTCH
Ciprofloxacin in Dextrose (5%) Injection
200 mg in 100 mL 5% Dextrose
Rx only (2 mg/mL) 100 mL
N 103 0069439519 0
INFUSE OVER A PERIOD OF 60 MINUTES
Each 100 mL contains:200mg Ciprofloxacin, 5 g Dextrose Hydrous, Lactic Acid (added as solubilizer), Water for Injection, USP; pH adjusted with Hydrochloric acid. pH 3.5 to 4.6.
Sterile, nonpyrogenic. Single dose flexible plastic container. Drug additves should not be made to this solution
Dosage: Intravenously as directed by a physician. See directions.
Attention Pharmacist: Dilspense the accompanying Medication Guide to each patient.
Cautions:Check for minute leaks by squeezing the inner bag firmly. If leaks are found, discard solution as sterility may be impaired
Must not be used in series connections. Do not use unless solution is clear. No further diIution is necessary.
Recommended storage: Store between (5-25°C) (41°-77°F). Avoid excessive heat. Protect from freezing. Protect from light. See package insert.
Do not remove unit from overwrap until ready to use. Do not use if overwrap has been previously opened or damaged. The overwrap is a moisture and light barrier. The inner bag maintains the sterility of the product.
M.L. No.: GUJ/DRUGS/G/LVP-5
Disctributed by
Pfizer Labs
Division of Pfizer, Inc, NY, NY, 10017
1000004444
Made in India

PRINCIPAL DISPLAY PANEL
TO OPEN - TEAR AT NOTCH
Ciprofloxacin in Dextrose (5%) Injection
400 mg in 200 mL 5% Dextrose
Rx only (2 mg/mL) 200 mL
N 10300694396272
INFUSE OVER A PERIOD OF 60 MINUTES
Each 100 mL contains:200mg Ciprofloxacin, 5 g Dextrose Hydrous, Lactic Acid (added as solubilizer), Water for Injection, USP; pH adjusted with Hydrochloric acid. pH 3.5 to 4.6.
Sterile, nonpyrogenic. Single dose flexible plastic container. Drug additves should not be made to this solution
Dosage: Intravenously as directed by a physician. See directions.
Attention Pharmacist: Dilspense the accompanying Medication Guide to each patient.
Cautions:Check for minute leaks by squeezing the inner bag firmly. If leaks are found, discard solution as sterility may be impaired
Must not be used in series connections. Do not use unless solution is clear. No further diIution is necessary.
Recommended storage: Store between (5-25°C) (41°-77°F). Avoid excessive heat. Protect from freezing. Protect from light. See package insert.
Do not remove unit from overwrap until ready to use. Do not use if overwrap has been previously opened or damaged. The overwrap is a moisture and light barrier. The inner bag maintains the sterility of the product.
M.L. No.: GUJ/DRUGS/G/LVP-5
Disctributed by
Pfizer Labs
Division of Pfizer, Inc, NY, NY, 10017
1000004447
Made in India

PRINCIPAL DISPLAY PANEL
Ciprofloxacin Injection, USP
200 mg/20 mL
(10 mg/mL)
For intravenous (I.V.) Infusion
SINGLE DOSE VIAL contains:
20 mL sterile 1% solution
200 mg ciprofloxacin
DILUTE BEFORE USE
Rx Only

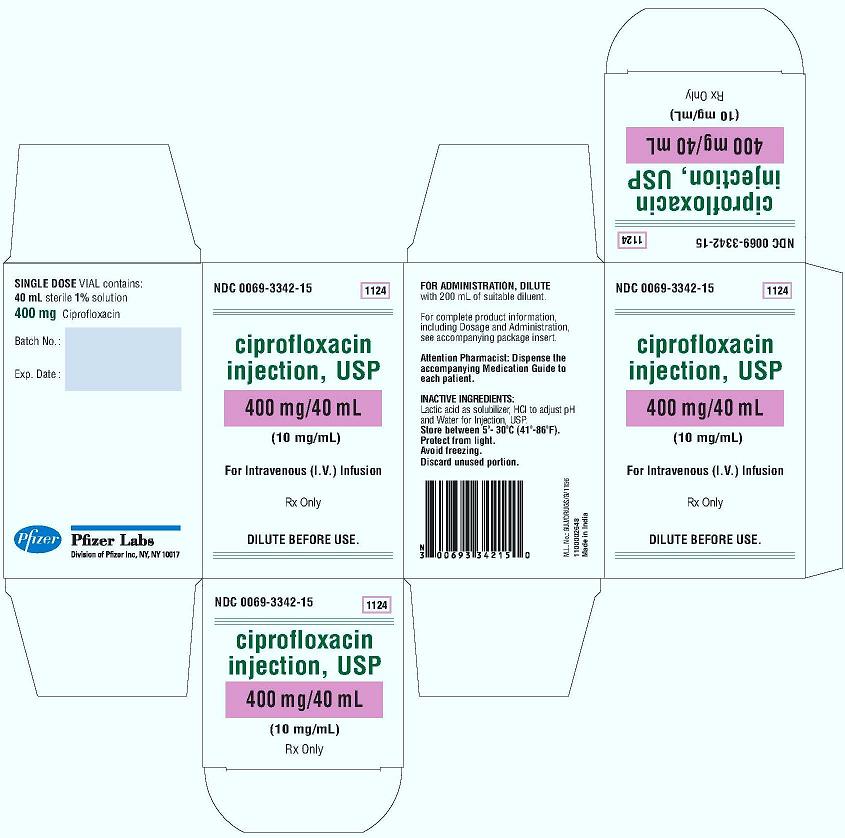
PRINCIPAL DISPLAY PANEL
NDC 0069-3241-15
11 22
Ciprofloxacin Injection, USP
200 mg/ 20 mL
(10 mg/mL)
For intravenous (I.V.) Infusion
Rx Only
DILUTE BEFORE USE
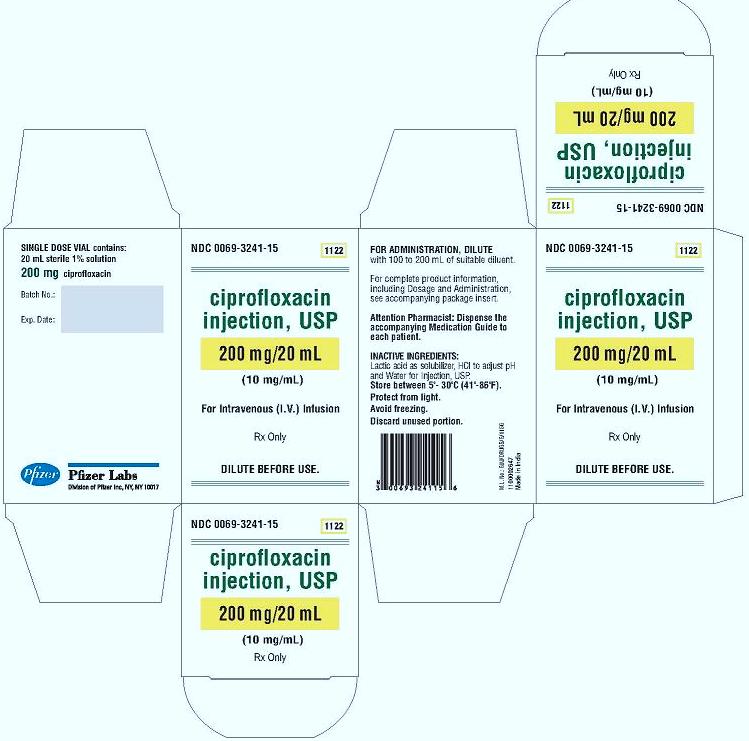
PRINCIPAL DISPLAY PANEL
Ciprofloxacin Injection, USP
400 mg/40 mL
(10 mg/mL)
For intravenous (I.V.) Infusion
SINGLE DOSE VIAL contains:
40 mL sterile 1% solution
400 mg ciprofloxacin
DILUTE BEFORE USE
Rx Only
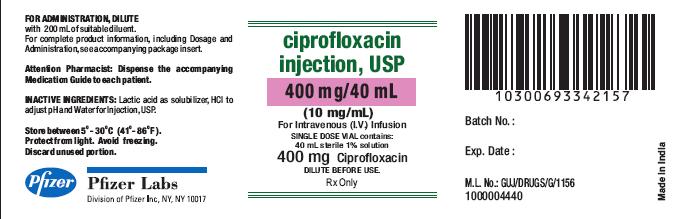
PRINCIPAL DISPLAY PANEL
NDC 0069-3342-15
11 22
Ciprofloxacin Injection, USP
400 mg/ 40 mL
(10 mg/mL)
For intravenous (I.V.) Infusion
Rx Only
DILUTE BEFORE USE