NDC Code(s) : 0009-5190-01, 0009-5190-02, 0009-5190-03, 0009-5190-04, 0009-5191-01, 0009-5191-02, 0009-5191-03, 0009-5191-04, 0009-5191-99
Packager : Pharmacia and Upjohn Company LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Detrol LAtolterodine tartrate CAPSULE, EXTENDED RELEASE | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Detrol LAtolterodine tartrate CAPSULE, EXTENDED RELEASE | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| LABELER - Pharmacia and Upjohn Company LLC(618054084) |
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 30 Capsule 2 mg Bottle Label
NDC 0009-5190-01
30 Capsules
Rx only
Detrol® LA
tolterodine tartrate
extended release capsules
2 mg
Pfizer
Distributed by
Pharmacia & Upjohn Co
Division of Pfizer Inc, NY, NY 10017

PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 2 mg Capsule Dose Foil Pack
Detrol
® LA
tolterodine tartrate
extended release
Capsule
2 mg
Pharmacia & Upjohn Co
Div of Pfizer Inc, NY, NY 10017
13803100

PRINCIPAL DISPLAY PANEL
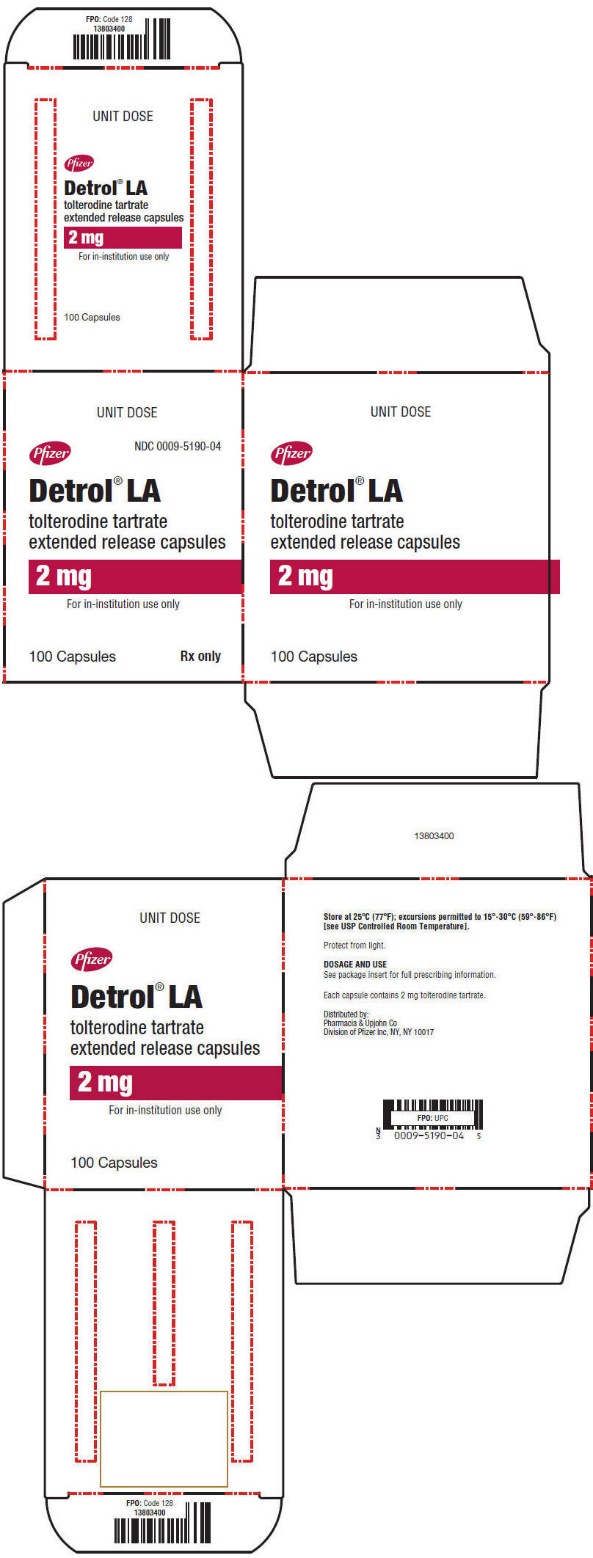
PRINCIPAL DISPLAY PANEL - 2 mg Capsule Unit Dose Carton
UNIT DOSE
Pfizer
NDC 0009-5190-04
Detrol
® LA
tolterodine tartrate
extended release capsules
2 mg
For in-institution use only
100 Capsules
Rx only
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 30 Capsule 4 mg Bottle Label
NDC 0009-5191-01
30 Capsules
Rx only
Detrol® LA
tolterodine tartrate
extended release capsules
4 mg
Pfizer
Distributed by
Pharmacia & Upjohn Co
Division of Pfizer Inc, NY, NY 10017

PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 4 mg Capsule Dose Foil Pack
Detrol
® LA
tolterodine tartrate
extended release
Capsule
4 mg
Pharmacia & Upjohn Co
Div of Pfizer Inc, NY, NY 10017
13802700

PRINCIPAL DISPLAY PANEL
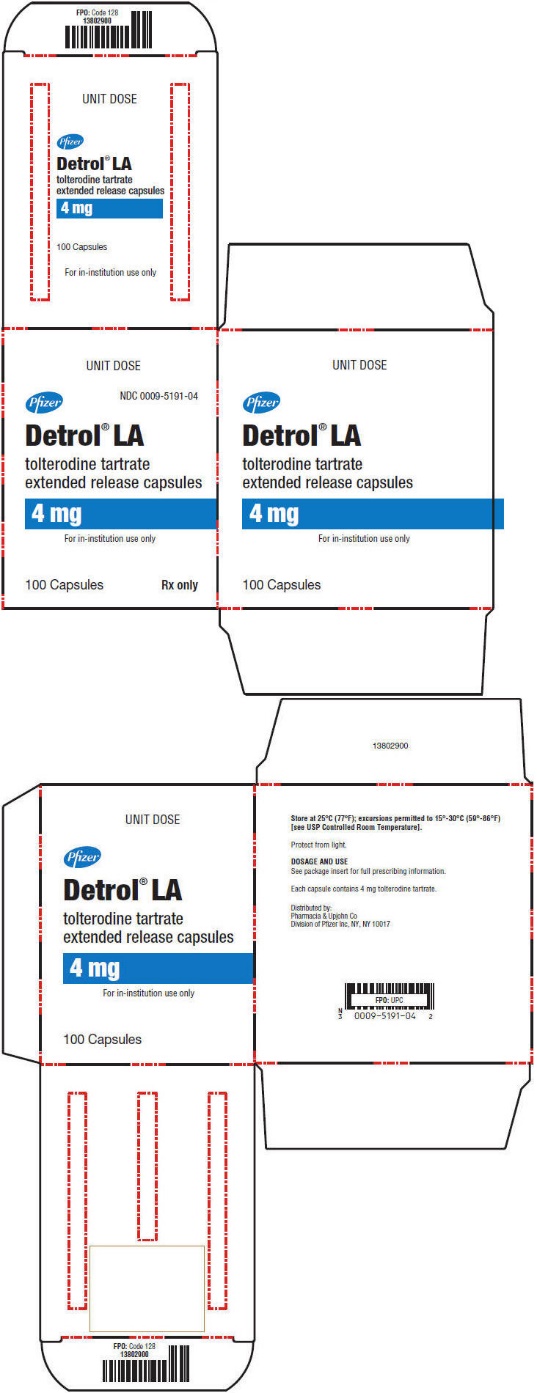
PRINCIPAL DISPLAY PANEL - 4 mg Capsule Unit Dose Carton
UNIT DOSE
Pfizer
NDC 0009-5191-04
Detrol
® LA
tolterodine tartrate
extended release capsules
4 mg
For in-institution use only
100 Capsules
Rx only
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 4 mg Capsule Sample Dose Pack
NDC 0009-5191-99
Detrol
®
LA
tolterodine tartrate
extended release capsules
4 mg
Detrol
®
LA
tolterodine tartrate
extended release capsules
4 mg
PROFESSIONAL
SAMPLE –
NOT FOR SALE
Protect from light
Detrol
®
LA
tolterodine tartrate
extended release capsules
4 mg
Manufactured by:
Pfizer Pharmaceuticals LLC, Puerto Rico
Pfizer
Distributed by
Pharmacia & Upjohn Co
Division of Pfizer Inc, NY, NY 10017
11604800
Lot
Exp

PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 4 mg Capsule Sample Carton
SEE ENCLOSED EDUCATIONAL BROCHURE
FOR MORE INFORMATION
PROFESSIONAL SAMPLE – NOT FOR SALE
NDC 0009-5191-99
Rx only
Detrol
®
LA
tolterodine tartrate
extended release capsules
4 mg
7 capsules
FOR FREE INFORMATION, CALL 1-888-846-9034
OR VISIT OUR WEB SITE www.detrolLA.com










