NDC Code(s) : 0009-0274-01, 0009-0280-02, 0009-0280-51, 0009-0280-03, 0009-0280-52, 0009-0306-02, 0009-0306-12
Packager : Pharmacia & Upjohn Company LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Depo-Medrolmethylprednisolone acetate INJECTION, SUSPENSION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Depo-Medrolmethylprednisolone acetate INJECTION, SUSPENSION | |||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Depo-Medrolmethylprednisolone acetate INJECTION, SUSPENSION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - Pharmacia & Upjohn Company LLC(618054084) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Pharmacia & Upjohn Company LLC | 618054084 | ANALYSIS(0009-0274, 0009-0280, 0009-0306), MANUFACTURE(0009-0274, 0009-0280, 0009-0306), API MANUFACTURE(0009-0274, 0009-0280, 0009-0306), PACK(0009-0274, 0009-0280, 0009-0306), LABEL(0009-0274, 0009-0280, 0009-0306) | |
PRINCIPAL DISPLAY PANEL
5 mL Multidose Vial
NDC 0009-0274-01
Depo-Medrol®
(methylPREDNISolone acetate
injectable suspension, USP)
100 mg/5 mL
(20 mg/mL)
Rx only

PRINCIPAL DISPLAY PANEL
5 mL
Multidose Vial
NDC 0009-0274-01
Depo-Medrol®
(methylPREDNISolone
acetate injectable
suspension, USP)
100 mg/5 mL
(20 mg/mL)
For intramuscular,
intrasynovial and
soft tissue injection only
NOT for IV use
Contains Benzyl Alcohol
as a Preservative
Rx only
Pfizer Injectables

PRINCIPAL DISPLAY PANEL
5 mL Multidose Vial
NDC 0009-0280-02
Depo-Medrol®
(methylPREDNISolone acetate
injectable suspension, USP)
200 mg/5 mL
(40 mg/mL)
Rx only

PRINCIPAL DISPLAY PANEL
25—5 mL Multidose Vials
NDC 0009-0280-51
Contains 25 of NDC 0009-0280-02
Depo-Medrol
®
(methylPREDNISolone acetate injectable suspension, USP)
200 mg/5 mL
(40 mg/mL)
For intramuscular, intrasynovial and soft tissue injection only
NOT for IV use
Contains Benzyl Alcohol as a Preservative
Pfizer Injectables
Rx only

PRINCIPAL DISPLAY PANEL
5 mL Multidose Vial
NDC 0009-0306-02
Depo-Medrol®
(methylPREDNISolone acetate
injectable suspension, USP)
400 mg/5 mL
(80 mg/mL)
Rx only

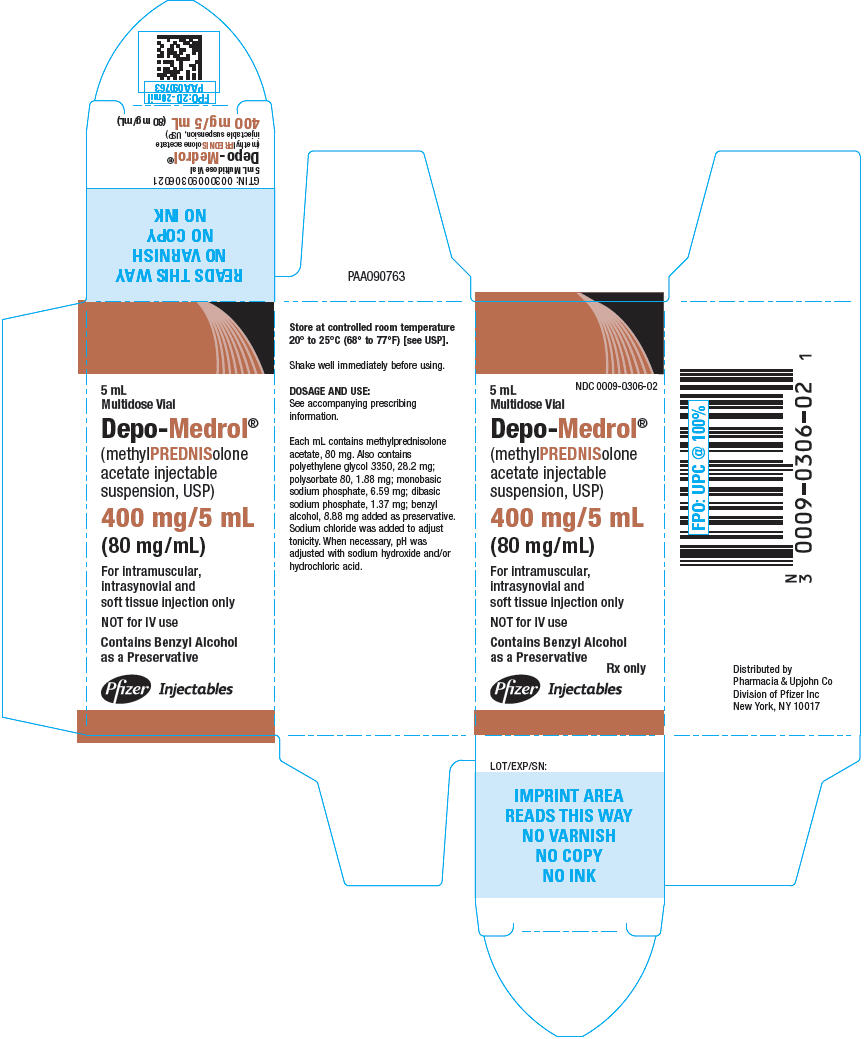
PRINCIPAL DISPLAY PANEL
5 mL
Multidose Vial
NDC 0009-0306-02
Depo-Medrol®
(methylPREDNISolone
acetate injectable
suspension, USP)
400 mg/5 mL
(80 mg/mL)
For intramuscular,
intrasynovial and
soft tissue injection only
NOT for IV use
Contains Benzyl Alcohol
as a Preservative
Rx only
Pfizer Injectables