NDC Code(s) : 0009-0039-33, 0009-0039-32, 0009-0047-27, 0009-0047-26
Packager : Pharmacia and Upjohn Company LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Solu-Medrolmethylprednisolone sodium succinate INJECTION, POWDER, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Solu-Medrolmethylprednisolone sodium succinate INJECTION, POWDER, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
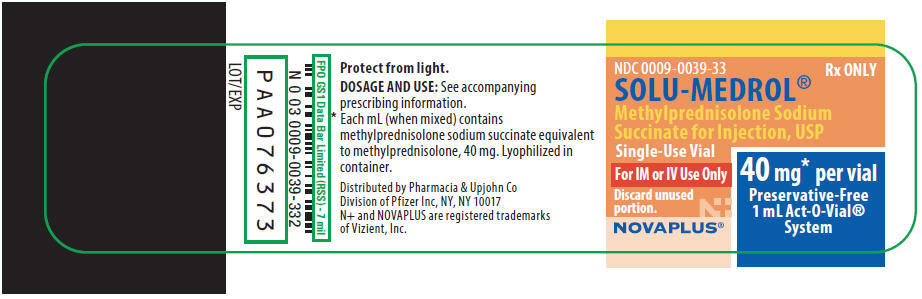
PRINCIPAL DISPLAY PANEL
NDC 0009-0039-33
Rx ONLY
SOLU-MEDROL®
Methylprednisolone Sodium
Succinate for Injection, USP
Single-Use Vial
For IM or IV Use Only
Discard unused
portion.
40 mg* per vial
Preservative-Free
1 mL Act-O-Vial®
System
NOVAPLUS®
PRINCIPAL DISPLAY PANEL
NDC 0009-0039-32
Contains 25 of NDC 0009-0039-33
SOLU-MEDROL®
Methylprednisolone Sodium
Succinate for Injection, USP
40 mg* per vial
Preservative-Free
25–1 mL Act-O-Vial®
Systems
For Intramuscular or Intravenous Use Only
Single-Use Vials
Discard unused portion.
Rx ONLY
NOVAPLUS®

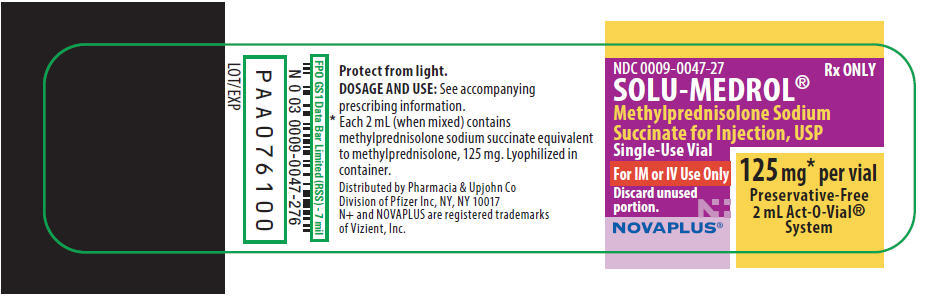
PRINCIPAL DISPLAY PANEL
NDC 0009-0047-27
Rx ONLY
SOLU-MEDROL®
Methylprednisolone Sodium
Succinate for Injection, USP
Single-Use Vial
For IM or IV Use Only
Discard unused
portion.
125 mg* per vial
Preservative-Free
2 mL Act-O-Vial®
System
NOVAPLUS®
PRINCIPAL DISPLAY PANEL
NDC 0009-0047-26
Contains 25 of NDC 0009-0047-27
SOLU-MEDROL®
Methylprednisolone Sodium
Succinate for Injection, USP
125 mg* per vial
Preservative-Free
25–2 mL Act-O-Vial®
Systems
For Intramuscular or Intravenous Use Only
Single-Use Vials
Discard unused portion.
Rx ONLY
NOVAPLUS®









