NDC Code(s) : 0007-3230-01, 0007-3230-02, 0007-3230-11, 0007-3230-61, 0007-3232-11, 0007-3234-01, 0007-3234-02, 0007-3234-11, 0007-3234-61, 0007-3236-01, 0007-3236-02, 0007-3236-11
Packager : GlaxoSmithKline LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| ARIXTRAfondaparinux sodium INJECTION, SOLUTION | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| ARIXTRAfondaparinux sodium INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ARIXTRAfondaparinux sodium INJECTION, SOLUTION | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| ARIXTRAfondaparinux sodium INJECTION, SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Principal Display Panel
NDC 0007-3230-02
Arixtra®
(fondaparinux sodium) Injection
2.5 mg/0.5 mL
2 x 0.5 mL
Contains 2 Single Dose, Prefilled Syringes
Rx only
Single Dose, Prefilled Syringes
Affixed with an Automatic Needle Protection System
For Subcutaneous Injection
Contents: Each single dose prefilled syringe contains 2.5 mg of fondaparinux sodium in 0.5 mL of an isotonic solution of sodium chloride and water for injection.
The needle guard of the prefilled syringe of ARIXTRA contains dry natural latex rubber that may cause allergic reactions in latex sensitive individuals.
Recommended Dose: 2.5 mg subcutaneous injection, once daily. See package insert.
Storage: Store at 25oC (77oF); excursions permitted to 15oC – 30oC (59oF -86oF), (see USP Controlled Room Temperature).
GlaxoSmithKline
Research Triangle Park, NC 27709
Made in France
©2008, GlaxoSmithKline
Rev. 6/08
11000000002826

PRINCIPAL DISPLAY PANEL
Principal Display Panel
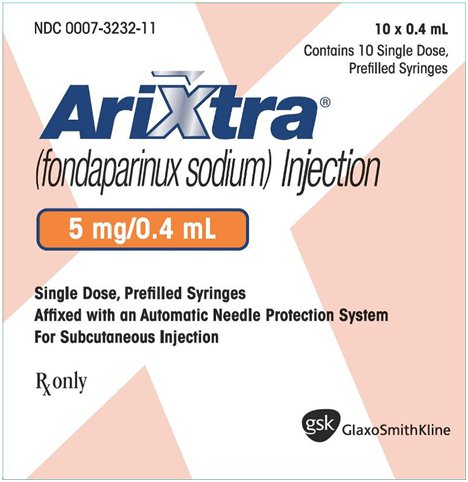
NDC 0007-3232-11
Arixtra®
(fondaparinux sodium) Injection
5 mg/0.4 mL
10 x 0.4 mL
Contains 10 Single Dose, Prefilled Syringes
Single Dose, Prefilled Syringes
Affixed with an Automatic Needle Protection System
For Subcutaneous Injection
Rx only
Made in France
©2011, GlaxoSmithKline
Rev. 8/11
11000000008395
PRINCIPAL DISPLAY PANEL
Principal Display Panel
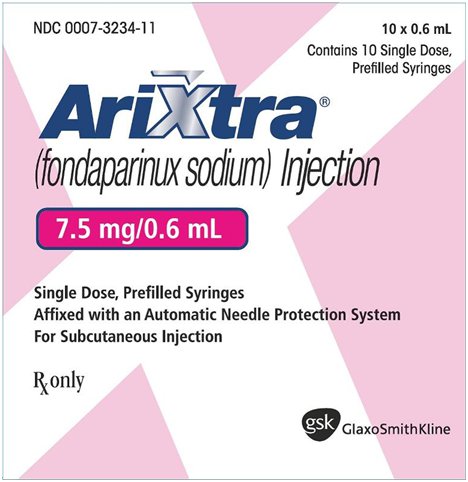
NDC 0007-3234-11
Arixtra®
(fondaparinux sodium) Injection
7.5 mg/0.6 mL
10 x 0.6 mL
Contains 10 Single Dose, Prefilled Syringes
Single Dose, Prefilled Syringes
Affixed with an Automatic Needle Protection System
For Subcutaneous Injection
Rx only
Made in France
©2011, GlaxoSmithKline
Rev. 8/11
11000000008394
PRINCIPAL DISPLAY PANEL
Principal Display Panel
NDC 0007-3236-11
Arixtra®
(fondaparinux sodium) Injection
10 mg/0.8 mL
10 x 0.8 mL
Contains 10 Single Dose, Prefilled Syringes
Single Dose, Prefilled Syringes
Affixed with an Automatic Needle Protection System
For Subcutaneous Injection
Rx only
Made in France
©2011, GlaxoSmithKline
Rev. 2/11
11000000007790










