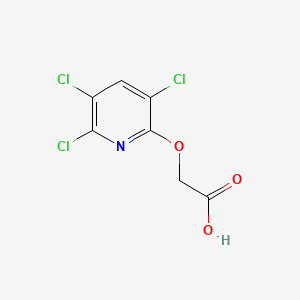

1. 3,5,6-trichloro-2-pyridyloxyacetic Acid
2. Garlon
3. Triclopyr Butoxyethyl Ester
4. Triclopyr Triethylamine Salt
5. Triclopyr-(3,5,6-trichloro-2-pyridl-oxyacetic Acid)
6. Triclopyr-triethylammonium
1. 55335-06-3
2. Garlon
3. Redeem
4. Garlon 2
5. Trichlopyr
6. Confront
7. Dowco 233
8. Turflon
9. Garlon 250
10. 3,5,6-trichloro-2-pyridyloxyacetic Acid
11. 2-((3,5,6-trichloropyridin-2-yl)oxy)acetic Acid
12. 2-(3,5,6-trichloropyridin-2-yl)oxyacetic Acid
13. Nsc 190671
14. ((3,5,6-trichloro-2-pyridinyl)oxy)acetic Acid
15. Acetic Acid, [(3,5,6-trichloro-2-pyridinyl)oxy]-
16. [(3,5,6-trichloropyridin-2-yl)oxy]acetic Acid
17. Mv06phj6i0
18. Chebi:9682
19. Triclopyr 10 Microg/ml In Acetone
20. Triclopyr 100 Microg/ml In Acetone
21. Acetic Acid, ((3,5,6-trichloro-2-pyridinyl)oxy)-
22. Nsc-190671
23. Ncgc00163927-04
24. Triclopyr 100 Microg/ml In Acetonitrile
25. Grazon Et
26. ((3,5,6-trichloropyridin-2-yl)oxy)acetic Acid
27. [(3,5,6-trichloro-2-pyridinyl)oxy]acetic Acid
28. 2-[(3,5,6-trichloropyridin-2-yl)oxy]acetic Acid
29. Triclopyr [ansi]
30. Caswell No. 882i
31. Remedy
32. Triclopyr [iso]
33. Release [pesticide]
34. Hsdb 7060
35. Triclopyr [ansi:iso]
36. Einecs 259-597-3
37. Unii-mv06phj6i0
38. Acetic Acid, 2-((3,5,6-trichloro-2-pyridinyl)oxy)-
39. Acetic Acid, 2-[(3,5,6-trichloro-2-pyridinyl)oxy]-
40. Epa Pesticide Chemical Code 116001
41. Brn 0225301
42. Triclopyr [mi]
43. 3,4,5-trichloro-2-pyridinyloxyacetic Acid
44. Triclopyr [hsdb]
45. Acetic Acid, (3,5,6-trichloro-2-pyridyloxy)-
46. Dsstox_cid_12497
47. Dsstox_rid_78957
48. 2-[(3,5,6-trichloro-2-pyridyl)oxy]acetic Acid
49. Dsstox_gsid_32497
50. Schembl37162
51. 4-21-00-00362 (beilstein Handbook Reference)
52. Chembl1865925
53. Dtxsid0032497
54. Zinc900723
55. Hy-b2051
56. Wln: T6nj Bo1vq Cg Eg Fg
57. Tox21_400073
58. Mfcd00072514
59. Nsc190671
60. Triclopyr 100 Microg/ml In Methanol
61. Akos015895897
62. Ac-2622
63. Ks-5287
64. Triclopyr 1000 Microg/ml In Methanol
65. Ncgc00163927-01
66. Ncgc00163927-02
67. Ncgc00163927-03
68. Ncgc00163927-05
69. Cas-55335-06-3
70. Db-052715
71. Acetic Acid,5,6-trichloro-2-pyridyl)oxy]-
72. Cs-0014145
73. Ft-0630678
74. T3742
75. [(3,6-trichloro-2-pyridinyl)oxy]acetic Acid
76. Acetic Acid,5,6-trichloro-2-pyridinyl)oxy]-
77. Triclopyr, Pestanal(r), Analytical Standard
78. D92737
79. 2-(3,5,6-trichloropyridin-2-yloxy)acetic Acid
80. [(3,5,6-trichloropyridin-2-yl)oxy]ethanoic Acid
81. 335t063
82. Acetic Acid, ((3,5,6-trichloro-2-pyridyl)oxy)-
83. Q2303660
84. W-105569
85. 2-((3,5,6-trichloro-2-pyridinyl)oxy)acetic Acid
86. Sbk
| Molecular Weight | 256.5 g/mol |
|---|---|
| Molecular Formula | C7H4Cl3NO3 |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 254.925676 g/mol |
| Monoisotopic Mass | 254.925676 g/mol |
| Topological Polar Surface Area | 59.4 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 216 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Herbicides
Pesticides used to destroy unwanted vegetation, especially various types of weeds, grasses (POACEAE), and woody plants. Some plants develop HERBICIDE RESISTANCE. (See all compounds classified as Herbicides.)
In the province of Quebec (Canada), the phytocide Garlon 4, whose active ingredient is triclopyr, is often used to prevent trees from reaching electrical conductors. The object of this paper is to assess the potential health risks in workers coming into contact with Garlon 4. Ten workers collected their urine during the 22 hr following the beginning of a work shift. Measured urinary amounts of triclopyr varied between 1 and 13 mg. The absorbed daily doses were estimated from the amounts of triclopyr in urine through the use of a kinetic model that links the rates of triclopyr elimination to absorbed doses. These estimated doses were compared with the no-observed-effect level (NOEL) observed in rats: 5 mg per kg of body weight. The upper-bound estimations of the worker's daily absorbed doses were found to be 13.3% or less of the rat NOEL.
PMID:15703284 Gosselin NH et al; Ann Occup Hyg 49 (5): 415-22 (2005)
Two herbicides, 2,4-D and triclopyr esters (application ratio 1.6:1 acid equivalents) were applied as a tank mix by a crew of 8 backpack sprayer applicators, a mixer/loader, and a field supervisor. The crew was employed in a conifer release program in northern California during the summer of 2002. Biomonitoring (urine, 24 hr) utilized 2,4-D and triclopyr (a.e.) as rapidly excreted exposure biomarkers. The absorbed dosages of 2,4-D and triclopyr were calculated based upon cotton whole body suits and biomonitoring. Dosages based upon accumulation of the herbicides on body suits averaged 42.6 ug (a.e.) 2,4-D/kg-d and 8.0 ug (a.e.) triclopyr/kg-d. Six consecutive days of concurrent urine collections showed that backpack applicators excreted an average of 11.0 ug (a.e.) 2,4-D/kg-d and 18.9 ug (a.e.) triclopyr/kg-d. Estimates based upon curve fitting were 17.1 and 29.3 ug (a.e.)/kg-d, respectively. Results suggest that passive dosimetry for 2,4-D consistently overestimated the dosage measured using biomonitoring by a factor of 2-3 fold, while for triclopyr, passive dosimetry underestimated the absorbed dose based on biomonitoring by a factor of 2-4 fold.
PMID:21500074 Zhang X et al; J Environ Sci Health B 46 (4): 281-93 (2011)
Disposition and metabolism of (14)C-triclopyr acid (98.8% a.i.) was investigated in male and female rats at a low oral dose (3 mg/kg), repeated low oral doses (3 mg/kg x 14 days), and a high dose (60 mg/kg). Comparison of disposition data in intravenously dosed and orally dosed rats demonstrated that triclopyr was well absorbed after oral administration. Excretion was relatively rapid at the low dose, with a majority of radioactivity eliminated in the urine by 24 hours. At 60 mg/kg, urinary elimination of (14)C-triclopyr derived radioactivity was decreased in male and female rats from 0-12 hours, due to apparent saturation of renal elimination mechanisms. Fecal elimination of (14)C-triclopyr derived radioactivity was a minor route of excretion, as was elimination via exhaled air. No significant effect was observed on metabolism or disposition of (14)C-triclopyr from repeated low oral dosing in male or female rats. Residual (14)C-triclopyr derived radioactivity was minimal in all dose groups, but measurable levels of tissue radioactivity were detected in perirenal fat of both sexes and ovaries of female rats which apparently increased with dose. Thus, potential accumulation of (14)C-triclopyr derived radioactivity may occur in these tissues. Urinary metabolites of (14)C-triclopyr were isolated and identified by HPLC and GC/MS. Unmetabolized parent chemical represented >90% of urinary radioactivity, with the remainder accounted for by the metabolite 3,5,6-trichloro-2-pyridinol (3,5,6-TCP), and possible glucuronide and/or sulfate conjugates of 3,5,6-TCP. Plasma elimination following intravenous administration of (14)C-triclopyr was consistent with a one-compartment model with an elimination half-life of 3.6 hr and zero-order kinetics from 0-12 hours at the 60 mg/kg dose. Kinetic parameters were optimized using SIMUSOLV modeling software. The model showed an apparent "flip-flop" phenomenon, in which absorption at the 3 mg/kg dose was rate limiting in elimination of (14)C-triclopyr derived radioactivity, but renal excretion was saturated and therefore limiting in elimination of (14)C-triclopyr derived radioactivity at the 60 mg/kg dose.
USEPA; Office of Prevention, Pesticides and Toxic Substances; Reregistration Eligibility Decision (RED) for Triclopyr p.16 EPA738-R-98-011 (October 1998). Available from, as of December 20, 2016: https://www.epa.gov/pesticides/reregistration/status.htm
Blood levels and urinary excretion of triclopyr, the active ingredient in Garlon herbicides, were followed in six volunteers given single oral doses of 0.1 and 0.5 mg/kg body weight. Five of these volunteers later received dermal applications of Garlon 4 herbicide formulation equivalent to 3.7 mg triclopyr/kg body weight applied to the forearm. Following oral administration blood levels peaked at 2-3 hr and declined to undetectable levels within 48 hr; more than 80% of the dose was found as unchanged triclopyr in the urine. An average of 1.37% of the applied dose was recovered in the urine; when corrected for recovery after oral administration this was equivalent to an absorption of 1.65%. Triclopyr is slowly absorbed through skin and is rapidly eliminated. It has very low potential to accumulate in man or to be absorbed through the skin in acutely toxic amounts.
PMID:2591984 Carmichael NG, et al; Hum Toxicol 8 (6): 431-437 (1989)
... 3,5,6-trichloro-2-pyridinol (3,5,6-TCP) ... is the primary analyte found in /human/ urine as a result of exposure to ... triclopyr.
PMID:10563869 Shackelford DD, et al; J Agric Food Chem 47(1): 177-182 (1999)
Disposition and metabolism of (14)C-triclopyr acid (98.8% a.i.) was investigated in male and female rats at a low oral dose (3 mg/kg), repeated low oral doses (3 mg/kg x 14 days), and a high dose (60 mg/kg). ... Urinary metabolites of (14)C-triclopyr were isolated and identified by HPLC and GC/MS. Unmetabolized parent chemical represented >90% of urinary radioactivity, with the remainder accounted for by the metabolite 3,5,6-trichloro-2-pyridinol (3,5,6-TCP), and possible glucuronide and/or sulfate conjugates of 3,5,6-TCP. ...
USEPA; Office of Prevention, Pesticides and Toxic Substances; Reregistration Eligibility Decision (RED) for Triclopyr p.16 EPA738-R-98-011 (October 1998). Available from, as of December 20, 2016: https://www.epa.gov/pesticides/reregistration/status.htm
Disposition and metabolism of (14)C-triclopyr acid (98.8% a.i.) was investigated in male and female rats at a low oral dose (3 mg/kg), repeated low oral doses (3 mg/kg x 14 days), and a high dose (60 mg/kg). ... Plasma elimination following intravenous administration of (14)C-triclopyr was consistent with a one-compartment model with an elimination half-life of 3.6 hr ... .
USEPA; Office of Prevention, Pesticides and Toxic Substances; Reregistration Eligibility Decision (RED) for Triclopyr p.16 EPA738-R-98-011 (October 1998). Available from, as of December 20, 2016: https://www.epa.gov/pesticides/reregistration/status.htm
A two-compartment pharmacokinetic model was used to describe the time-course of triclopyr clearance; half-lives for the rapid initial and slower terminal phases were 1.3 hr and 5.1 hr respectively, and were independent of dose. Due to the slow half-life for dermal absorption (t1/2 = 16.8 hr) the rapid initial elimination phase was obscured and the pharmacokinetics could be simplified by a one-compartment model.
PMID:2591984 Carmichael NG, et al; Hum Toxicol 8 (6): 431-437 (1989)