
1. 24729-96-2
2. St075182
3. Dtxsid80860321
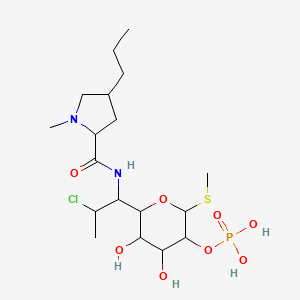
4. Methyl 7-chloro-6,7,8-trideoxy-6-[(1-methyl-4-propylprolyl)amino]-2-o-phosphono-1-thiooctopyranoside
5. Akos015960484
6. Ncgc00389304-01
7. Ac-11380
8. Methyl7-chloro-6,7,8-trideoxy-6-(1-methyl-trans-4-propyl-l-2-pyrrolidinecarboxamido)-1-thio-l-threo-alpha-d-galacto-octopyranoside2-(dihydrogenphosphate)
9. Db-046536
10. Ft-0603210
| Molecular Weight | 505.0 g/mol |
|---|---|
| Molecular Formula | C18H34ClN2O8PS |
| XLogP3 | -1.2 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 9 |
| Exact Mass | 504.1462019 g/mol |
| Monoisotopic Mass | 504.1462019 g/mol |
| Topological Polar Surface Area | 174 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 658 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 9 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 21 | |
|---|---|
| Drug Name | CLEOCIN |
| Active Ingredient | CLINDAMYCIN PHOSPHATE |
| Company | PHARMACIA AND UPJOHN (Application Number: N050537); PHARMACIA AND UPJOHN (Application Number: N050680); PHARMACIA AND UPJOHN (Application Number: N050767. Patent: 6495157) |
| 2 of 21 | |
|---|---|
| Drug Name | CLINDA-DERM |
| Active Ingredient | CLINDAMYCIN PHOSPHATE |
| Company | PADDOCK LLC (Application Number: A063329) |
| 3 of 21 | |
|---|---|
| Drug Name | CLINDAGEL |
| Active Ingredient | CLINDAMYCIN PHOSPHATE |
| Company | PRECISION DERMAT (Application Number: N050782. Patent: 6387383) |
| 4 of 21 | |
|---|---|
| Drug Name | CLINDAMYCIN PHOSPHATE IN 0.9% SODIUM CHLORIDE |
| Active Ingredient | CLINDAMYCIN PHOSPHATE |
| Company | BAXTER HLTHCARE CORP (Application Number: N208083) |
| 5 of 21 | |
|---|---|
| Drug Name | CLINDAMYCIN PHOSPHATE AND BENZOYL PEROXIDE |
| Active Ingredient | BENZOYL PEROXIDE; CLINDAMYCIN PHOSPHATE |
| Company | ACTAVIS LABS UT INC (Application Number: A205128); MYLAN PHARMS INC (Application Number: A065443); PERRIGO ISRAEL (Application Number: A090979); PERRIGO ISRAEL (Application Number: A202440); TARO (Application Number: A206218); TOLMAR (Application Number: A203688); TOLMAR (Application Number: A204087) |
| 6 of 21 | |
|---|---|
| Drug Name | BENZACLIN |
| Active Ingredient | BENZOYL PEROXIDE; CLINDAMYCIN PHOSPHATE |
| Company | VALEANT BERMUDA (Application Number: N050756) |
| 7 of 21 | |
|---|---|
| Drug Name | ACANYA |
| Active Ingredient | BENZOYL PEROXIDE; CLINDAMYCIN PHOSPHATE |
| Company | DOW PHARM (Application Number: N050819. Patents: 8288434, 8663699, 8895070, 9078870, 9504704, 9561208) |
| 8 of 21 | |
|---|---|
| Drug Name | CLEOCIN PHOSPHATE |
| Active Ingredient | CLINDAMYCIN PHOSPHATE |
| Company | PHARMACIA AND UPJOHN (Application Number: A062803); PHARMACIA AND UPJOHN (Application Number: N050441) |
| 9 of 21 | |
|---|---|
| Drug Name | CLINDAMYCIN PHOSPHATE IN DEXTROSE 5% |
| Active Ingredient | CLINDAMYCIN PHOSPHATE |
| Company | ABRAXIS PHARM (Application Number: N050635) |
| 10 of 21 | |
|---|---|
| Drug Name | CLINDESSE |
| Active Ingredient | CLINDAMYCIN PHOSPHATE |
| Company | PERRIGO PHARMA INTL (Application Number: N050793. Patents: 6899890, 9789057) |
| 11 of 21 | |
|---|---|
| Drug Name | ONEXTON |
| Active Ingredient | BENZOYL PEROXIDE; CLINDAMYCIN PHOSPHATE |
| Company | DOW PHARM (Application Number: N050819. Patents: 8288434, 8663699, 8895070, 9078870, 9504704, 9561208) |
| 12 of 21 | |
|---|---|
| Drug Name | CLINDAMYCIN PHOSPHATE AND TRETINOIN |
| Active Ingredient | CLINDAMYCIN PHOSPHATE; TRETINOIN |
| Company | ACTAVIS MID ATLANTIC (Application Number: A202564) |
| 13 of 21 | |
|---|---|
| Drug Name | ZIANA |
| Active Ingredient | CLINDAMYCIN PHOSPHATE; TRETINOIN |
| Company | MEDICIS (Application Number: N050802. Patent: 6387383) |
| 14 of 21 | |
|---|---|
| Drug Name | EVOCLIN |
| Active Ingredient | CLINDAMYCIN PHOSPHATE |
| Company | MYLAN PHARMS INC (Application Number: N050801. Patents: 7141237, 7374747) |
| 15 of 21 | |
|---|---|
| Drug Name | CLINDETS |
| Active Ingredient | CLINDAMYCIN PHOSPHATE |
| Company | PERRIGO CO (Application Number: A064136) |
| 16 of 21 | |
|---|---|
| Drug Name | DUAC |
| Active Ingredient | BENZOYL PEROXIDE; CLINDAMYCIN PHOSPHATE |
| Company | STIEFEL (Application Number: N050741) |
| 17 of 21 | |
|---|---|
| Drug Name | VELTIN |
| Active Ingredient | CLINDAMYCIN PHOSPHATE; TRETINOIN |
| Company | AQUA PHARMS LLC (Application Number: N050803) |
| 18 of 21 | |
|---|---|
| Drug Name | CLEOCIN PHOSPHATE IN DEXTROSE 5% IN PLASTIC CONTAINER |
| Active Ingredient | CLINDAMYCIN PHOSPHATE |
| Company | PHARMACIA AND UPJOHN (Application Number: N050639) |
| 19 of 21 | |
|---|---|
| Drug Name | CLEOCIN T |
| Active Ingredient | CLINDAMYCIN PHOSPHATE |
| Company | PHARMACIA AND UPJOHN (Application Number: N050537); PHARMACIA AND UPJOHN (Application Number: N050600); PHARMACIA AND UPJOHN (Application Number: N050615) |
| 20 of 21 | |
|---|---|
| Drug Name | CLINDAMYCIN PHOSPHATE IN 5% DEXTROSE IN PLASTIC CONTAINER |
| Active Ingredient | CLINDAMYCIN PHOSPHATE |
| Company | AKORN INC (Application Number: A203048); BAXTER HLTHCARE CORP (Application Number: A208084); SANDOZ INC (Application Number: A201692) |
| 21 of 21 | |
|---|---|
| Drug Name | CLINDAMYCIN PHOSPHATE |
| Active Ingredient | CLINDAMYCIN PHOSPHATE |
| Company | AKORN (Application Number: A065513); ALVOGEN INC (Application Number: A062800); ALVOGEN INC (Application Number: A062801); ALVOGEN INC (Application Number: A062943); FOUGERA PHARMS INC (Application Number: A064159); FOUGERA PHARMS (Application Number: A064160); FOUGERA PHARMS (Application Number: A065067); FOUGERA PHARMS (Application Number: A065139); FOUGERA PHARMS (Application Number: A065254); FRESENIUS KABI USA (Application Number: A065346); FRESENIUS KABI USA (Application Number: A065347); G AND W LABS INC (Application Number: A062811); GLASSHOUSE PHARMS (Application Number: A209846); MYLAN LABS LTD (Application Number: A204748); MYLAN LABS LTD (Application Number: A204749); PERRIGO NEW YORK (Application Number: A064050); PERRIGO NEW YORK (Application Number: A065049); PERRIGO UK FINCO (Application Number: A090785); SAGENT PHARMS (Application Number: A090108); SAGENT PHARMS (Application Number: A090109); TARO PHARM INDS (Application Number: A065184); TELIGENT PHARMA INC (Application Number: A206945); VINTAGE PHARMS (Application Number: A203343); WEST-WARD PHARMS INT (Application Number: A062889); WEST-WARD PHARMS INT (Application Number: A065206); WOCKHARDT (Application Number: A063304) |