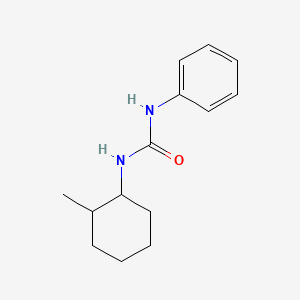

1. 1-(2-methylcyclohexyl)-3-phenylurea
1. 1-(2-methylcyclohexyl)-3-phenylurea
2. 1982-49-6
3. Tupersan
4. Trey
5. Urea, N-(2-methylcyclohexyl)-n'-phenyl-
6. Du Pont Herbicide 1,318
7. Du Pont 1318
8. N-phenyl-n'-(2-methylcyclohexyl)urea
9. Urea, 1-(2-methylcyclohexyl)-3-phenyl-
10. 1-(2-methylcycohexyl)-3-phenylurea
11. H 1318
12. Nsc 131951
13. Chebi:81744
14. 513s964ljo
15. Nsc-131951
16. Urea,n-(2-methylcyclohexyl)-n'-phenyl-
17. Caswell No. 733a
18. Dupont 1318
19. Siduron [ansi:bsi:iso]
20. Siduron [iso]
21. Hsdb 1764
22. Einecs 217-844-2
23. Epa Pesticide Chemical Code 035509
24. N-(2-methylcyclohexyl)-n'-phenylurea
25. Unii-513s964ljo
26. Siduron [mi]
27. Dsstox_cid_12474
28. Dsstox_rid_78952
29. Dsstox_gsid_32474
30. Schembl66323
31. Wln: L6tj Amvmr& B1
32. Chembl1902120
33. Dtxsid7032474
34. Tox21_301425
35. Nsc131951
36. Akos001192124
37. Ncgc00163937-01
38. Ncgc00163937-02
39. Ncgc00255363-01
40. N-(2-methylcyclohexyl)-n'-phenylurea #
41. Cas-1982-49-6
42. Siduron, Pestanal(r), Analytical Standard
43. C18435
44. N-(2-methylcyclohexyl)-n'-phenylurea [hsdb]
45. Q27155591
| Molecular Weight | 232.32 g/mol |
|---|---|
| Molecular Formula | C14H20N2O |
| XLogP3 | 3.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 2 |
| Exact Mass | 232.157563266 g/mol |
| Monoisotopic Mass | 232.157563266 g/mol |
| Topological Polar Surface Area | 41.1 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 249 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Most readily absorbed through root system; less so through foliage and stems. ... Experimental evidence indicates that Siduron is translocated in the xylem.
Weed Science Society of America. Herbicide Handbook. 5th ed. Champaign, Illinois: Weed Science Society of America, 1983., p. 427
Urine from a dog receiving 2500 ppm siduron in the diet was collected and analyzed for the parent compound and metabolites using a colorimetric method, and mass, IR, and NMR spectrometry, and a metabolic pathway was proposed; none of the parent compound was found, but three glucuronide conjugates of hydroxysubstituted siduron were isolated and identified, one substituted on the phenyl ring, one substituted on the cyclohexyl ring, and another substituted in both locations; it appears that siduron is first hydroxylated at either location, then both of these mono-hydroxylated compounds are hydroxylated again to form 1-(4-hydroxy-2-methylcyclo-hexyl)-3-p-hydroxyphenylurea; comparison of urine from the dog with that from a rat on a similar 2500 ppm diet, showed that the rat formed slightly less of the cyclohexyl-substituted compound than the dog; 800-1000 ppm of total siduron metabolites was bound in the urine of these two animals.
California Environmental Protection Agency/Department of Pesticide Regulation; Toxicology Data Review Summary for Siduron (1982-49-6). Available from, as of May 13, 2009: https://www.cdpr.ca.gov/docs/risk/toxsums/toxsumlist.htm
In studies with (14)C-labeled siduron, no metabolites ... were detectable in barley plants after an 8 day absorption period.
Weed Science Society of America. Herbicide Handbook. 5th ed. Champaign, Illinois: Weed Science Society of America, 1983., p. 427
... Both phenyl and cyclohexyl rings are subjected to hydroxylation in the dog. ... The pathways proposed ... show that siduron is either hydroxylated at para position of phenyl nucleus or at 4 position of 2-methylcyclohexyl moiety. Both metabolites are further hydroxylated to form 1-(4-hydroxy-2-methylcyclohexyl)-3-(p-hydroxyphenyl)urea.
Kearney, P.C., and D. D. Kaufman (eds.) Herbicides: Chemistry, Degredation and Mode of Action. Volumes 1 and 2. 2nd ed. New York: Marcel Dekker, Inc., 1975., p. 246
When urine samples of both dogs and rats were subjected to hydrolysis and analyzed for aminophenol /as well as/ aniline, it was observed that hydroxylation of the phenyl moiety was more prominent in the rat than in dog. Apparently rats exhibit particular efficiency in hydroxylating the aromatic nucleus of phenylureas. In another study in which (14)C- carbonyl-labeled compound was applied ... microbial degradation was major route of siduron disappearance in soil. Metabolites separated on thin-layer plates had same characteristics as those identified in urine, and /it was/ ... concluded that ring hydroxylation was also operative in soil environment.
Kearney, P.C., and D. D. Kaufman (eds.) Herbicides: Chemistry, Degredation and Mode of Action. Volumes 1 and 2. 2nd ed. New York: Marcel Dekker, Inc., 1975., p. 246
For more Metabolism/Metabolites (Complete) data for SIDURON (6 total), please visit the HSDB record page.
Unlike other substituted urea herbicides, siduron is not a potent inhibitor of photosynthesis; phytotoxic symptoms appear to be associated with root growth inhibition.
Weed Science Society of America. Herbicide Handbook. 5th ed. Champaign, Illinois: Weed Science Society of America, 1983., p. 427
