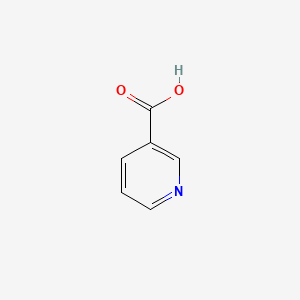

1. 3 Pyridinecarboxylic Acid
2. 3-pyridinecarboxylic Acid
3. Aluminum Salt, Niacin
4. Enduracin
5. Hydrochloride, Niacin
6. Induracin
7. Lithium Nicotinate
8. Niacin
9. Niacin Aluminum Salt
10. Niacin Ammonium Salt
11. Niacin Calcium Salt
12. Niacin Cobalt (2+) Salt
13. Niacin Copper (2+) Salt
14. Niacin Hydrochloride
15. Niacin Iron (2+) Salt
16. Niacin Lithium Salt
17. Niacin Lithium Salt, Hemihydrate
18. Niacin Magnesium Salt
19. Niacin Manganese (2+) Salt
20. Niacin Potassium Salt
21. Niacin Sodium Salt
22. Niacin Tartrate
23. Niacin Tosylate
24. Niacin Zinc Salt
25. Nicamin
26. Nico 400
27. Nico-400
28. Nico400
29. Nicobid
30. Nicocap
31. Nicolar
32. Nicotinate
33. Nicotinate, Lithium
34. Potassium Salt, Niacin
35. Sodium Salt, Niacin
36. Tartrate, Niacin
37. Tosylate, Niacin
38. Wampocap
1. Niacin
2. 59-67-6
3. Pyridine-3-carboxylic Acid
4. 3-pyridinecarboxylic Acid
5. 3-carboxypyridine
6. Wampocap
7. Niaspan
8. Acidum Nicotinicum
9. Vitamin B3
10. Nicolar
11. Apelagrin
12. Pellagrin
13. Akotin
14. Daskil
15. Efacin
16. Pelonin
17. Linic
18. Nicamin
19. Nicobid
20. Nicocap
21. Enduracin
22. Nicodelmine
23. Niconacid
24. Nicotinipca
25. Pellagramin
26. Direktan
27. Nicacid
28. Nicangin
29. Peviton
30. Bionic
31. Diacin
32. Nicyl
33. Nyclin
34. Niac
35. Vitaplex N
36. Davitamon Pp
37. Nico-span
38. Tega-span
39. Nicocidin
40. Nicocrisina
41. Niconazid
42. Nicoside
43. Nicotamin
44. Nicotene
45. Nicovasan
46. Nicovasen
47. Nipellen
48. Sk-niacin
49. Naotin
50. Niacor
51. Nicodon
52. Niconat
53. Nicosan 3
54. Nicosyl
55. Nicotil
56. Tinic
57. 3-carboxylpyridine
58. Nicotine Acid
59. Slo-niacin
60. Nico
61. 3-picolinic Acid
62. Nicotinsaure
63. Nico-400
64. Acide Nicotinique
65. Pyridine-beta-carboxylic Acid
66. Nicagin
67. Anti-pellagra Vitamin
68. Caswell No. 598
69. Pp Factor
70. Kyselina Nikotinova
71. P.p. Factor
72. Pellagra Preventive Factor
73. S115
74. Nicotinsaure [german]
75. Acido Nicotinico
76. 3-pyridylcarboxylic Acid
77. M-pyridinecarboxylic Acid
78. Kyselina Nikotinova [czech]
79. Mfcd00006391
80. Niacine
81. Ccris 1902
82. Pyridine-carboxylique-3
83. Epa Pesticide Chemical Code 056701
84. Acide Nicotinique [inn-french]
85. Acido Nicotinico [inn-spanish]
86. Acidum Nicotinicum [inn-latin]
87. Hsdb 3134
88. Pyridine-carboxylique-3 [french]
89. Ai3-18994
90. Pyridinecarboxylic Acid, 3-
91. Niacin [usp]
92. Sr 4390
93. Brn 0109591
94. Niacin Extended Release
95. Nicotinic Acid [inn]
96. Nah
97. Chembl573
98. Beta-pyridinecarboxylic Acid
99. Nsc-169454
100. Mls000069603
101. Pyridine-.beta.-carboxylic Acid
102. Chebi:15940
103. Niacin (usp)
104. 2679mf687a
105. P.p. Factor-pellagra Preventive Factor
106. Cas-59-67-6
107. Ncgc00016268-02
108. Smr000059024
109. Dsstox_cid_932
110. [5, 6-3h]-niacin
111. Dsstox_rid_75875
112. Dsstox_gsid_20932
113. Niacin [usan]
114. Nicotinicacid
115. Nio
116. Niacin (nicotinic Acid)
117. Sr-01000722017
118. 36321-41-2
119. Einecs 200-441-0
120. Niaspan Titration Starter Pack
121. Nsc 169454
122. Pellagra
123. Nikotinsaeure
124. Ncotnc Acd
125. Unii-2679mf687a
126. Preventative Factor
127. Antipellagra Vitamin
128. Niaspan (tn)
129. 3pyrcooh
130. [3h]nicotinic Acid
131. Niacor (tn)
132. Nicotinic Acid,(s)
133. [3h]-nicotinic Acid
134. Spectrum_001063
135. 3-pyridinecarboxylicacid
136. Nicotinic Acid, Ph Eur
137. Niacin [vandf]
138. Niacin [hsdb]
139. Niacin [inci]
140. 5-pyridinecarboxylic Acid
141. Niacin [fcc]
142. Niacin [usp-rs]
143. Opera_id_1346
144. Prestwick0_000881
145. Prestwick1_000881
146. Prestwick2_000881
147. Prestwick3_000881
148. Pyridine-3-carbonic Acid
149. Spectrum2_000006
150. Spectrum3_000515
151. Spectrum4_000965
152. Spectrum5_001287
153. Vitamin B-3
154. 3-pyridyl Carboxylic Acid
155. Nicotinic Acid-d3(major)
156. Wln: T6nj Cvq
157. 3-pyridine Carboxylic Acid
158. Bmse000104
159. Nicotinic Acid, >=98%
160. Nicotinic Acid, Usp Grade
161. Ec 200-441-0
162. Schembl1433
163. Nicotinic Acid [mi]
164. Oprea1_514398
165. Vitamin B3 [vandf]
166. Bspbio_000662
167. Bspbio_002069
168. Kbiogr_001309
169. Kbioss_001543
170. Niacin [orange Book]
171. Nicotinic Acid [jan]
172. 5-22-02-00057 (beilstein Handbook Reference)
173. Bidd:gt0644
174. Divk1c_000695
175. Nicotinic Acid (jp17/inn)
176. Simcor Component Niacin
177. Spectrum1500430
178. .beta.-pyridinecarboxylic Acid
179. Spbio_000011
180. Spbio_002881
181. Advicor Component Niacin
182. Niacin [usp Monograph]
183. Nicotinic Acid [vandf]
184. Bpbio1_000730
185. Gtpl1588
186. Gtpl1594
187. Zinc1795
188. Nicotinic Acid [mart.]
189. Dtxsid1020932
190. Nicotinic Acid [who-dd]
191. Nicotinic Acid [who-ip]
192. Bdbm23515
193. Hms502c17
194. Kbio1_000695
195. Kbio2_001543
196. Kbio2_004111
197. Kbio2_006679
198. Kbio3_001569
199. Abt-919
200. Niacin Component Of Simcor
201. Nicotinic Acid [ema Epar]
202. Ninds_000695
203. Hms1570b04
204. Hms1920p17
205. Hms2091h22
206. Hms2097b04
207. Hms2236a05
208. Hms3259k21
209. Hms3371e07
210. Hms3655k08
211. Hms3714b04
212. Niacin Component Of Advicor
213. Pharmakon1600-01500430
214. Nicotinic Acid, Analytical Standard
215. Bcp16301
216. Hy-b0143
217. Str00112
218. Tox21_110337
219. Tox21_201420
220. Tox21_302904
221. Ac8691
222. Bbl037343
223. Ccg-38340
224. Nicotinic Acid [ep Impurity]
225. Nicotinic Acid, For Synthesis, 99%
226. Nsc169454
227. Nsc757241
228. S1744
229. Stk301803
230. Nicotinic Acid (vitamin B3) Solution
231. Nicotinic Acid [ep Monograph]
232. Akos000118980
233. Nicotinic Acid, >=99.5% (hplc)
234. Tox21_110337_1
235. Am81316
236. Cs-1946
237. Db00627
238. Nc00524
239. Nicotinic Acid 1.0 Mg/ml In Methanol
240. Nsc-757241
241. Ps-4255
242. Sdccgmls-0066610.p001
243. Idi1_000695
244. Ncgc00016268-01
245. Ncgc00016268-03
246. Ncgc00016268-04
247. Ncgc00016268-05
248. Ncgc00016268-08
249. Ncgc00016268-09
250. Ncgc00094734-01
251. Ncgc00094734-02
252. Ncgc00256537-01
253. Ncgc00258971-01
254. Ac-22484
255. Acidum Nicotinicum [who-ip Latin]
256. Bp-21419
257. Nci60_001041
258. Nicotinic Acid, Nist(r) Srm(r) 148
259. Nicotinic Acid, Plant Cell Culture Tested
260. Sy011111
261. Nicotinic Acid 100 Microg/ml In Methanol
262. Sbi-0051456.p003
263. Db-007765
264. Nicotinic Acid [matrix For Maldi-tof/ms]
265. Ab00052050
266. Ft-0600004
267. Ft-0672715
268. Ft-0672716
269. Ft-0672717
270. Ft-0672718
271. Ft-0773496
272. N0082
273. N1103
274. Nicotinic Acid 10 Microg/ml In Acetonitrile
275. Nicotinic Acid, Purum, >=99.0% (hplc)
276. Sw197229-3
277. C00253
278. D00049
279. Nicotinic Acid, Saj Special Grade, >=99.5%
280. Ab00052050-13
281. Ab00052050_14
282. Ab00052050_15
283. Nicotinic Acid, Meets Usp Testing Specifications
284. Ac-907/25014105
285. L001199
286. Methyl Nicotinate Impurity A [ep Impurity]
287. Nicotinic Acid, Vetec(tm) Reagent Grade, >=98%
288. Q134658
289. J-523605
290. Sr-01000722017-2
291. Sr-01000722017-3
292. Sr-01000722017-4
293. Z56755709
294. 3ddb011e-f3a6-45b6-a2d2-77b2a6e8936e
295. F2191-0082
296. Niacin, United States Pharmacopeia (usp) Reference Standard
297. Nicotinic Acid, Certified Reference Material, Tracecert(r)
298. Nicotinic Acid, European Pharmacopoeia (ep) Reference Standard
299. Nicotinic Acid, Matrix Substance For Maldi-ms, >=99.5% (hplc)
300. Nicotinic Acid, For Inorganic Trace Analysis, >=99.999% (metals Basis)
301. Niacin (nicotinic Acid), Pharmaceutical Secondary Standard; Certified Reference Material
302. 101113-41-1
303. Nicotinic Acid (vitamin B3) Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
304. Nicotinic Acid, Bioreagent, Suitable For Cell Culture, Suitable For Insect Cell Culture, Suitable For Plant Cell Culture, >=98%
| Molecular Weight | 123.11 g/mol |
|---|---|
| Molecular Formula | C6H5NO2 |
| XLogP3 | 0.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 123.032028402 g/mol |
| Monoisotopic Mass | 123.032028402 g/mol |
| Topological Polar Surface Area | 50.2 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 114 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Niacin |
| PubMed Health | Niacin (By mouth) |
| Drug Classes | Antihyperlipidemic, Nutriceutical, Nutritive Agent |
| Drug Label | NIASPAN (niacin tablet, film-coated extended-release), contains niacin, which at therapeutic doses is an antihyperlipidemic agent. Niacin (nicotinic acid, or 3-pyridinecarboxylic acid) is a white, crystalline powder, very soluble in water, with the f... |
| Active Ingredient | Niacin |
| Dosage Form | Tablet, extended release; Tablet |
| Route | Oral |
| Strength | 500mg; 750mg; 1gm |
| Market Status | Prescription |
| Company | Wockhardt; Lupin; Sun Pharma Global; Barr |
| 2 of 6 | |
|---|---|
| Drug Name | Niacor |
| Drug Label | Niacin or nicotinic acid, a water-soluble B-complex vitamin and antihyperlipidemic agent, is 3-pyridinecarboxylic acid. It is a white, crystalline powder, sparingly soluble in water. It has the following structural formula:Each NIACOR Tablet, for o... |
| Active Ingredient | Niacin |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Upsher Smith |
| 3 of 6 | |
|---|---|
| Drug Name | Niaspan |
| Drug Label | NIASPAN (niacin tablet, film-coated extended-release), contains niacin, which at therapeutic doses is an antihyperlipidemic agent. Niacin (nicotinic acid, or 3-pyridinecarboxylic acid) is a white, crystalline powder, very soluble in water, with the f... |
| Active Ingredient | Niacin |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 500mg; 750mg; 1gm |
| Market Status | Prescription |
| Company | Abbvie |
| 4 of 6 | |
|---|---|
| Drug Name | Niacin |
| PubMed Health | Niacin (By mouth) |
| Drug Classes | Antihyperlipidemic, Nutriceutical, Nutritive Agent |
| Drug Label | NIASPAN (niacin tablet, film-coated extended-release), contains niacin, which at therapeutic doses is an antihyperlipidemic agent. Niacin (nicotinic acid, or 3-pyridinecarboxylic acid) is a white, crystalline powder, very soluble in water, with the f... |
| Active Ingredient | Niacin |
| Dosage Form | Tablet, extended release; Tablet |
| Route | Oral |
| Strength | 500mg; 750mg; 1gm |
| Market Status | Prescription |
| Company | Wockhardt; Lupin; Sun Pharma Global; Barr |
| 5 of 6 | |
|---|---|
| Drug Name | Niacor |
| Drug Label | Niacin or nicotinic acid, a water-soluble B-complex vitamin and antihyperlipidemic agent, is 3-pyridinecarboxylic acid. It is a white, crystalline powder, sparingly soluble in water. It has the following structural formula:Each NIACOR Tablet, for o... |
| Active Ingredient | Niacin |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Upsher Smith |
| 6 of 6 | |
|---|---|
| Drug Name | Niaspan |
| Drug Label | NIASPAN (niacin tablet, film-coated extended-release), contains niacin, which at therapeutic doses is an antihyperlipidemic agent. Niacin (nicotinic acid, or 3-pyridinecarboxylic acid) is a white, crystalline powder, very soluble in water, with the f... |
| Active Ingredient | Niacin |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 500mg; 750mg; 1gm |
| Market Status | Prescription |
| Company | Abbvie |
Hypolipidemic Agents, Vasodilator Agents, Vitamin B Complex
National Library of Medicine's Medical Subject Headings. Citalopram. Online file (MeSH, 2018). Available from, as of February 2, 2018: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Niacin included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of February 2, 2018: https://clinicaltrials.gov/
Niacin is used as an adjunct to dietary therapy in patients with a history of myocardial infarction (MI) and hypercholesterolemia to reduce the risk of recurrent nonfatal MI. Extended-release niacin in fixed combination with lovastatin is used in patients for whom treatment with both extended-release niacin and lovastatin is appropriate. /Included in US product label/
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 1936
Niacin, in combination with a bile acid sequestrant, also is used to slow the progression or promote regression of atherosclerosis in patients with clinical evidence of coronary heart disease who have elevated cholesterol concentrations. Extended-release niacin in fixed combination with lovastatin is used in patients for whom treatment with both extended-release niacin and lovastatin is appropriate. /Included in US product label/
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 1936
For more Therapeutic Uses (Complete) data for Nicotinic acid (31 total), please visit the HSDB record page.
Concomitant administration of niacin with alcohol, hot drinks, or spicy foods may increase the risk of flushing or pruritus; these beverages or foods should be avoided at the time of drug ingestion.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 1937
Decreased glucose tolerance has been reported in patients receiving niacin in controlled clinical trials. Therefore, the manufacturers and many clinicians suggest that patients with (or those at risk of developing) diabetes mellitus be observed closely. The ACC/AHA cholesterol management guideline recommends that fasting blood glucose concentrations or glycosylated hemoglobin (hemoglobin A1c, HbA1c) be obtained before initiation of therapy, following an increase in niacin dosage, and periodically (e.g., every 6 months) thereafter. If niacin is used in diabetic or potentially diabetic patients, dosages of niacin and/or antidiabetic agents should be adjusted appropriately. Niacin should be discontinued if hyperglycemia persists.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 1939
Niacin should be used with caution in patients with unstable angina, acute myocardial infarction (MI), or coronary heart disease (CHD), particularly when these patients also are receiving vasoactive drugs such as nitrates, calcium-channel blockers, or adrenergic blocking agents. Since niacin therapy has been associated with small, but statistically significant, increases in prothrombin time (PT), the drug, alone or in fixed combination with lovastatin, should be used with caution in patients undergoing surgery. PT and platelet count should be monitored closely in patients receiving niacin concomitantly with anticoagulants.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 1939
Because hyperuricemia has been reported with niacin therapy, the drug should be used with caution in patients predisposed to gout. The ACC/AHA cholesterol management guideline recommends that uric acid concentrations be obtained before initiation of therapy, following an increase in niacin dosage, and periodically (e.g., every 6 months) thereafter. Niacin should be discontinued if acute gout occurs.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 1939
For more Drug Warnings (Complete) data for Nicotinic acid (33 total), please visit the HSDB record page.
Niacin is indicated to prevent vitamin deficiencies in pediatric and adult patients receiving parenteral nutrition as part of multivitamin intravenous injections. Niacin oral tablets are indicated as a monotherapy or in combination with simvastatin or lovastatin to treat primary hyperlipidemia and mixed dyslipidemia. It can also be used to reduce the risk of nonfatal myocardial infarctions in patients with a history of myocardial infarction and hyperlipidemia. Niacin is also indicated with bile acid binding resins to treat atherosclerosis in patients with coronary artery disease and hyperlipidemia or to treat primary hyperlipidemia. Finally niacin is indicated to treat severe hypertriglyceridemia.
FDA Label
Niacin is a B vitamin used to treat vitamin deficiencies as well as hyperlipidemia, dyslipidemia, hypertriglyceridemia, and to reduce the risk of myocardial infarctions. Niacin acts to decrease levels of very low density lipoproteins and low density lipoproteins, while increasing levels of high density lipoproteins. Niacin has a wide therapeutic window with usual oral doses between 500mg and 2000mg. Patients with diabetes, renal failure, uncontrolled hypothyroidism, and elderly patients taking niacin with simvastatin or lovastatin are at increased risk of myopathy and rhabdomyolysis.
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Hypolipidemic Agents
Substances that lower the levels of certain LIPIDS in the BLOOD. They are used to treat HYPERLIPIDEMIAS. (See all compounds classified as Hypolipidemic Agents.)
Vitamin B Complex
A group of water-soluble vitamins, some of which are COENZYMES. (See all compounds classified as Vitamin B Complex.)
C - Cardiovascular system
C04 - Peripheral vasodilators
C04A - Peripheral vasodilators
C04AC - Nicotinic acid and derivatives
C04AC01 - Nicotinic acid
C - Cardiovascular system
C10 - Lipid modifying agents
C10A - Lipid modifying agents, plain
C10AD - Nicotinic acid and derivatives
C10AD02 - Nicotinic acid
Absorption
In patients with chronic kidney disease, the Cmax is 0.06g/mL for a 500mg oral dose, 2.42g/mL for a 1000mg oral dose, and 4.22g/mL for a 1500mg oral dose. The Tmax is 3.0 hours for a 1000mg or 1500mg oral dose. The AUC is 1.44g\*h/mL for a 500mg oral dose, 6.66g\*h/mL for a 1000mg oral dose, and 12.41g\*h/mL for a 1500mg oral dose. These values did not drastically differ in patients requiring dialysis.
Route of Elimination
69.5% of a dose of niacin is recovered in urine. 37.9% of the recovered dose was N-methyl-2-pyridone-5-carboxamide, 16.0% was N-methylnicotinamide, 11.6% was nicotinuric acid, and 3.2% was niacin.
Volume of Distribution
Data regarding the volume of distribution of niacin is not readily available.
Clearance
Data regarding the clearance of niacin is not readily available.
/MILK/ Niacin is distributed in human milk.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 1940
Niacin is rapidly and extensively (60-76% of dose) absorbed following oral administration. Peak plasma concentrations of niacin following administration of an immediate-release (Niacor) or extended-release (Niaspan) niacin preparation generally are attained within 30-60 minutes or 4-5 hours after oral administration, respectively. The bioavailability of 1 tablet containing 1 g of extended-release niacin in fixed combination with 40 mg of lovastatin (Advicor 1 g/40 mg) differs from that of 2 tablets each containing 500 mg of extended-release niacin in fixed combination with 20 mg of lovastatin (Advicor 500 mg/20 mg). ... Peak plasma concentrations of niacin and metabolites following oral administration of Niaspan extended-release tablets appear to be slightly higher in women than in men, possibly because of differences in metabolism. Limited data suggest that women may exhibit greater antilipemic response to niacin than men, possibly because of gender-specific differences in the metabolic rate or volume of distribution of the drug.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 1941
Niacin is distributed mainly to the liver, kidney, and adipose tissue.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 1941
Niacin and its metabolites are rapidly excreted in urine. Following oral administration of single and multiple doses of an immediate-release (Niacor) or extended-release (Niaspan) niacin preparation, approximately 88 or 60-76% of the dose, respectively, was excreted in urine as unchanged drug and inactive metabolites.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 1941
For more Absorption, Distribution and Excretion (Complete) data for Nicotinic acid (10 total), please visit the HSDB record page.
The metabolism of niacin is poorly described in the literature, but the metabolites niacinamide, niacinamide N-oxide, nicotinuric acid, N1-methyl-2-pyridone-5-carboxamide, N1-methyl-4-pyridone-5-carboxamide, and trigonelline have been identified in human urine.
... Nicotinamide is the main substance that is transported between the different tissues as a precursor of nicotinamide adenine dinucleotide (NAD) synthesis. The liver, kidneys, brain and erythrocytes prefer nicotinic acid as a precursor for NAD synthesis, but testes and ovaries prefer nicotinamide. NAD nucleosidase cleaves NAD with nicotinamide as one of the products. This can be deamidated to form nicotinic acid (and re-converted to NAD) or methylated and released via urine. Excretion of the amide (and its metabolites) tends to be more extensive compared to the acid.
Organization for Economic Cooperation and Development; Screening Information Data Set for 3-Pyridinecarboxamide (Nicotinamide) CAS #: 98-92-0 p.40 (2005). Available from, as of July 23, 2018: https://www.inchem.org/pages/sids.html
Niacin is rapidly metabolized and undergoes extensive first-pass metabolism. The drug is converted to several metabolites, including nicotinuric acid (NUA), nicotinamide, and nicotinamide adenine dinucleotide (NAD). At doses used to treat hyperlipoproteinemia, the principal metabolic pathways appear to be saturable, and niacin is thought to exhibit nonlinear, dose-dependent pharmacokinetics. Nicotinamide does not appear to exert antilipemic effects; the activity of other metabolites on lipoprotein fractions currently are unknown.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 1941
After doses of 100 mg of niacin (total dose of 200 mg), the urinary excretion of alkalihydrolyzable niacin derivatives and of N1-methylnicotinamide increased from 6.0 to 14.6 mg and from 2.8 to 5.7 mg after 3 hr in four human subjects, respectively. Chromatographic results showed the major metabolite in the urine to be nicotinuric acid, forming 92-99% of the alkalihydrolysable derivatives. Niacinamide (1-4%) was the other metabolite. There was no free niacin in the urine except in one subject who had intense flushing of the skin soon after ingesting the niacin. In these four subjects, there was a large increase in the excretion of N1-methylnicotinamide, which varied from 6.9 to 16.6 mg/3 hr. The small rise in the tertiary nicotinyl derivatives (0.9 to 1.8 mg) was solely due to niacinamide, since no other nicotinyl compound could be detected on the paper chromatograms. Urine from undosed subjects, averaged 0.53 mg N1-methylnicotinamide for a period of 3 hr. The total content of tertiary alkali-hydrolyzable derivative of niacin ranged from 0.2 to 0.3 mg within 3 hr.
Cosmetic Ingredient Review (CIR) Expert Panel; Final Report of the Safety Assessment of Niacinamide and Niacin; Intl J of Toxicology 24 (Suppl 5): p.7 (2005).
N1-methyl-4- pyridone-3-carboxamide is a major metabolite of niacin and niacinamide which has been found to be synthesized from N1- methylnicotinamide.
Cosmetic Ingredient Review (CIR) Expert Panel; Final Report of the Safety Assessment of Niacinamide and Niacin; Intl J of Toxicology 24 (Suppl 5): p.8 (2005). Available from, as of July 23, 218: https://www.cir-safety.org/ingredients
For more Metabolism/Metabolites (Complete) data for Nicotinic acid (8 total), please visit the HSDB record page.
The half life of niacin is 0.9h, nicotinuric acid is 1.3h, and nicotinamide is 4.3h.
/The authors/ describe a case of massive oral niacin overdose that resulted in severe persistent hypotension without the manifestation of cutaneous flushing. ... A 56-year-old male with a history of schizophrenia presented to the emergency department after orally ingesting 11,000 mg of niacin. ... Serum niacin levels were 8.2 ug/mL and 5.6 ug/mL at 48 and 96 hours post ingestion, respectively, giving an apparent half-life of 87 hours. ...
PMID:16496499 Mularski RA et al; Clin Toxicol (Phila) 44 (1): 81-4 (2006)
Half-life about 45 min.
Knoben, J.E. and P.O. Anderson (eds.) Handbook of Clinical Drug Data. 6th ed. Bethesda, MD: Drug Intelligence Publications, Inc. 1988., p. 502
The plasma half-life of niacin has been reported to range from 20-60 minutes.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 1941
Niacin performs a number of functions in the body and so has many mechanisms, not all of which have been fully described. Niacin can decrease lipids and apolipoprotein B (apo B)-containing lipoproteins by modulating triglyceride synthesis in the liver, which degrades apo B, or by modulating lipolysis in adipose tissue. Niacin inhibits hepatocyte diacylglycerol acyltransferase-2. This action prevents the final step of triglyceride synthesis in hepatocytes, limiting available triglycerides for very low density lipoproteins (VLDL). This activity also leads to intracellular degradation of apo B and decreased production of low density lipoproteins, the catabolic product of VLDL. Niacin also inhibits a high density lipoprotein (HDL) catabolism receptor, which increases the levels and half life of HDL.
Prolonged niacin treatment elicits beneficial effects on the plasma lipid and lipoprotein profile that is associated with a protective CVD risk profile. Acute niacin treatment inhibits nonesterified fatty acid release from adipocytes and stimulates prostaglandin release from skin Langerhans cells, but the acute effects diminish upon prolonged treatment, while the beneficial effects remain. To gain insight in the prolonged effects of niacin on lipid metabolism in adipocytes, we used a mouse model with a human-like lipoprotein metabolism and drug response [female APOE*3-Leiden.CETP (apoE3 Leiden cholesteryl ester transfer protein) mice] treated with and without niacin for 15 weeks. The gene expression profile of gonadal white adipose tissue (gWAT) from niacin-treated mice showed an upregulation of the "biosynthesis of unsaturated fatty acids" pathway, which was corroborated by quantitative PCR and analysis of the FA ratios in gWAT. Also, adipocytes from niacin-treated mice secreted more of the PUFA DHA ex vivo. This resulted in an increased DHA/arachidonic acid (AA) ratio in the adipocyte FA secretion profile and in plasma of niacin-treated mice. Interestingly, the DHA metabolite 19,20-dihydroxy docosapentaenoic acid (19,20-diHDPA) was increased in plasma of niacin-treated mice. Both an increased DHA/AA ratio and increased 19,20-diHDPA are indicative for an anti-inflammatory profile and may indirectly contribute to the atheroprotective lipid and lipoprotein profile associated with prolonged niacin treatment.
PMID:25320342 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4242446 Heemskerk MM et al; J Lipid Res 55 (12): 2532-40 (2014)
/The study objective was/ to determine the effects of niacin on adiponectin and markers of adipose tissue inflammation in a mouse model of obesity. Male C57BL/6 mice were placed on a control or high-fat diet (HFD) and were maintained on such diets for the duration of the study. After 6 weeks on the control or high fat diets, vehicle or niacin treatments were initiated and maintained for 5 weeks. Identical studies were conducted concurrently in HCA2 (-/-) (niacin receptor(-/-)) mice. Niacin increased serum concentrations of the anti-inflammatory adipokine, adiponectin by 21% in HFD-fed wild-type mice, but had no effect on lean wild-type or lean or HFD-fed HCA2 (-/-) mice. Niacin increased adiponectin gene and protein expression in the HFD-fed wild-type mice only. The increases in adiponectin serum concentrations, gene and protein expression occurred independently of changes in expression of PPARgamma C/EBPalpha or SREBP-1c (key transcription factors known to positively regulate adiponectin gene transcription) in the adipose tissue. Further, niacin had no effect on adipose tissue expression of ERp44, Ero1-Lalpha, or DsbA-L (key ER chaperones involved in adiponectin production and secretion). However, niacin treatment attenuated HFD-induced increases in adipose tissue gene expression of MCP-1 and IL-1beta in the wild-type HFD-fed mice. Niacin also reduced the expression of the pro-inflammatory M1 macrophage marker CD11c in HFD-fed wild-type mice. Niacin treatment attenuates obesity-induced adipose tissue inflammation through increased adiponectin and anti-inflammatory cytokine expression and reduced pro-inflammatory cytokine expression in a niacin receptor-dependent manner.
PMID:23967184 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3742781 Wanders D et al; PLoS One 8 (8): e71285 (2013)
Nicotinic acid (niacin), a vitamin of the B complex, has been used for almost 50 years as a lipid-lowering drug. The pharmacological effect of nicotinic acid requires doses that are much higher than those provided by a normal diet. Its primary action is to decrease lipolysis in adipose tissue by inhibiting hormone-sensitive triglyceride lipase. This anti-lipolytic effect of nicotinic acid involves the inhibition of cyclic adenosine monophosphate (cAMP) accumulation in adipose tissue through a G(i)-protein-mediated inhibition of adenylyl cyclase. A G-protein-coupled receptor for nicotinic acid has been proposed in adipocytes. Here, we show that the orphan G-protein-coupled receptor, "protein upregulated in macrophages by interferon-gamma" (mouse PUMA-G, human HM74), is highly expressed in adipose tissue and is a nicotinic acid receptor. Binding of nicotinic acid to PUMA-G or HM74 results in a G(i)-mediated decrease in cAMP levels. In mice lacking PUMA-G, the nicotinic acid-induced decrease in free fatty acid (FFA) and triglyceride plasma levels was abrogated, indicating that PUMA-G mediates the anti-lipolytic and lipid-lowering effects of nicotinic acid in vivo. The identification of the nicotinic acid receptor may be useful in the development of new drugs to treat dyslipidemia.
PMID:12563315 Tunaru S et al; Nat Med 9 (3): 352-5 (2003)