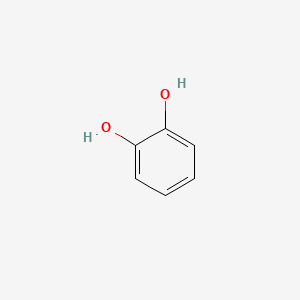

1. 1,2-benzenediol
2. 1,2-dihydroxybenzene
3. 1,3-dihydroxybenzene
4. 2-hydroxyphenol
5. Catechol Dipotassium Salt
6. Catechol Sodium Salt
7. Catechol, 14c-labeled Cpd
8. Pyrocatechol
1. Pyrocatechol
2. 120-80-9
3. 1,2-benzenediol
4. 1,2-dihydroxybenzene
5. Benzene-1,2-diol
6. Pyrocatechin
7. 2-hydroxyphenol
8. O-benzenediol
9. O-dihydroxybenzene
10. O-dioxybenzene
11. O-hydroquinone
12. O-hydroxyphenol
13. Phthalhydroquinone
14. Pyrocatechine
15. O-phenylenediol
16. Oxyphenic Acid
17. Fouramine Pch
18. Benzenediol
19. Durafur Developer C
20. Pelagol Grey C
21. Catechin (phenol)
22. Fourrine 68
23. Benzene, O-dihydroxy-
24. Catechol (phenol)
25. O-diphenol
26. C.i. Oxidation Base 26
27. Pyrokatechin
28. Pyrokatechol
29. Katechol
30. Ortho-dihydroxybenzene
31. Nci-c55856
32. Nsc 1573
33. C.i. 76500
34. Catechol-pyrocatechol
35. 12385-08-9
36. Mfcd00002188
37. Lf3aj089dq
38. 1,2-benzenediol, Homopolymer
39. Chembl280998
40. Dtxsid3020257
41. Chebi:18135
42. Nsc-1573
43. Ortho-hydroxyphenol
44. 26982-53-6
45. Caq
46. Dsstox_cid_257
47. Ortho-benzenediol
48. Ortho-dioxybenzene
49. Ortho-hydroquinone
50. Dsstox_rid_75468
51. Katechol [czech]
52. Ortho-phenylenediol
53. Pyrocatechinic Acid
54. Dsstox_gsid_20257
55. Pyrokatechin [czech]
56. Pyrokatechol [czech]
57. Benzene-1,2-diol (pyrocatechol)
58. Ci Oxidation Base 26
59. Phthalic Alcohol
60. Cas-120-80-9
61. Smr000326660
62. Ccris 741
63. Hsdb 1436
64. Einecs 204-427-5
65. Unii-lf3aj089dq
66. Brn 0471401
67. Oxyphenate
68. Ci 76500
69. Kachin
70. Ortho-diphenol
71. Benzene Diol
72. Ortho-quinol
73. Ai3-03995
74. 4oow
75. Alpha-hydroxyphenol
76. 1,2-benzenedio
77. O-dihydroxy-benzene
78. Phenol Derivative, 2
79. 3fw4
80. 4k7i
81. Catechol [hsdb]
82. Catechol [iarc]
83. Pyrocatechol, >=99%
84. Catechol [vandf]
85. Lopac-c-9510
86. Pyrocatechol [mi]
87. Wln: Qr Bq
88. Bmse000385
89. Ec 204-427-5
90. Pyrocatechol [inci]
91. 1,2-dihydroxybenzene, Xi
92. 1,2-benzenediol; Catechol
93. Lopac0_000280
94. Schembl18351
95. Mls002153385
96. Mls002303022
97. Bidd:er0327
98. Pyrocatechinic Acidpyrocatechol
99. Pyrocatechol, P.a., 99.0%
100. Bdbm26188
101. Durafur Developer Cfouramine Pch
102. Nsc1573
103. Hms2233a17
104. Hms3260h22
105. Hms3373k16
106. Tox21_202317
107. Tox21_300153
108. Tox21_500280
109. S6305
110. Stk398651
111. Zinc13512214
112. Akos000119002
113. Ccg-204375
114. Db02232
115. Lp00280
116. Sdccgsbi-0050268.p002
117. Catechol 100 Microg/ml In Acetonitrile
118. Ncgc00015283-01
119. Ncgc00015283-02
120. Ncgc00015283-03
121. Ncgc00015283-04
122. Ncgc00015283-05
123. Ncgc00015283-06
124. Ncgc00015283-07
125. Ncgc00015283-08
126. Ncgc00015283-10
127. Ncgc00091262-01
128. Ncgc00091262-02
129. Ncgc00091262-03
130. Ncgc00253952-01
131. Ncgc00259866-01
132. Ncgc00260965-01
133. Ac-34196
134. Bp-21156
135. Bs-20054
136. Catechol (pyrocatechol; Benzene-1,2-diol)
137. Db-003770
138. C.i.-76500
139. Eu-0100280
140. Ft-0606411
141. P0317
142. P0567
143. C 9510
144. C00090
145. C01785
146. D91943
147. 1,2-dihydroxybenzene, Reagentplus(r), >=99%
148. Pyrocatechol, Purified By Sublimation, >=99.5%
149. A804599
150. Ab-131/40235236
151. Q282440
152. Sr-01000075791
153. Sr-01000075791-1
154. W-109068
155. F0001-0332
156. Pyrocatechol, Certified Reference Material, Tracecert(r)
157. Z1262246103
158. Pyrocatechol, Plant Cell Culture Tested, Bioreagent, >=99%, Powder
159. 2h-1-benzopyran-3,5,7-triol, 2-(3,4-dihydroxyphenyl)-3,4-dihydro-,(2r-trans)-
| Molecular Weight | 110.11 g/mol |
|---|---|
| Molecular Formula | C6H6O2 |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 110.036779430 g/mol |
| Monoisotopic Mass | 110.036779430 g/mol |
| Topological Polar Surface Area | 40.5 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 62.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
4. =very toxic: Probable oral lethal dose (human) 50 to 500 mg/kg, between 1 teaspoon and 1 ounce for 70 kg person (150 lbs)
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-190
Pyrocatechol is readily absorbed from the GI tract and through the intact skin of mice, and probably through the lungs ... Part of the catechol ... conjugates in the body with glucuronic, sulfuric, and other acids and is excreted in the urine, with a little "free" pyrocatechol. The conjugates hydrolyze easily in the urine with the liberation of the "free" catechol, which is oxidized by air with the formation of dark-colored substances that impart to the urine a "smokey" appearance.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 4:400
When mice were exposed to cigarette smoke containing radiolabeled pyrocatechol, pyrocatechol was distributed readily into the blood and tissues; 90% of the radioactivity was excreted in the urine within 24 hr.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 4:400
Catechol... is absorbed in the respiratory tract. ...Very little is excreted in the urine as free catechol.
Sullivan, J.B., Krieger G.R. (eds). Clinical Environmental Health and Toxic Exposures. Second edition. Lippincott Williams and Wilkins, Philadelphia, Pennsylvania 1999., p. 1262
The "'S" skin notation in the listing /of the American Conference of Industrial Hygienists (ACGIH)/ refers to the "potential significant contribution to the overall exposure by the cutaneous route, including mucous membrane and the eyes, either by contact with vapors or, of probable greater significance, by direct skin contact with the substance."
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 4:402
Part of the catechol is oxidized with polyphenol oxidase to benzoquinone. Another fraction conjugates in the body with glucuronic, sulfuric, and other acids ... The conjugates hydrolyze easily in the urine with the liberation of the free catechol ...
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 4:400
Catechol yields guaiacol in rat. ... In the rabbit /catechol/ ... yields o-hydroxyphenyl-beta-d-glucuronide, o-hydroxyphenyl sulfate, and hydroxyquinol ... .
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. C-11
Structure-reactivity studies ... undertaken in rat ... results obtained with ... catechol ... show that 2 vicinal hydroxyl groups are a necessary condition for the /methylation/ reaction to take place, providing evidence for mode of action of catechol o-methyl transferase.
Testa, B. and P. Jenner. Drug Metabolism: Chemical & Biochemical Aspects. New York: Marcel Dekker, Inc., 1976., p. 174
Rabbits administered pyrocatechol orally excreted in the urine 18% as sulfate, 70% as monoglucuronide, and 2% as free pyrocatechol.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 4:400
For more Metabolism/Metabolites (Complete) data for CATECHOL (9 total), please visit the HSDB record page.
Catechol has known human metabolites that include Diphenol glucuronide, catechol sulfate, and o-Methoxyphenyl sulfate.
Catechol is a known human metabolite of phenol.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The calculated biological half-life of pyrocatechol in humans was 3-7 hr.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 4:401
The effects of catechol on various ionic channels of isolated primary afferent neurons of the bull frog were examined by a single suction electrode clamp system, which combined internal perfusion and current or voltage clamp using an electronic switching circuit. Catechol was found to inhibit rather specifically the fast potassium(+) current as does 4-aminopyridine. Calcium(2+), sodium(+) and slow potassium(+) currents were not affected. Although both 4-aminopyridine and catechol were inhibitors of the fast potassium(+) channels, their sites of action were quite different. Catechol was effective when applied on the external surface of the cell membrane whereas 4-aminopyridine acted preferably internally. We assumed that a single fast potassium(+) channel has two distinct sites for blockers: the catechol site is exposed to the external medium or situated at the outer orifice of the pore, and the 4-aminopyridine site is located within the same channel but is more easily accessible from inside the nerve cell than outside. The 4-aminopyridine and catechol sites were not, however, completely separate and independent of each other since a synergistic interaction was observed between catechol and 4-aminopyridine.
PMID:2427692 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1182528 Ito I, Maeno T; J Physiol 373: 115-27 (1986)
Catechol, a naturally occurring and an important industrial chemical, has been shown to have strong promotion activity and induce glandular stomach tumors in rodents. In addition, catechol is a major metabolite of carcinogenic benzene. To clarify the carcinogenic mechanism of catechol, we investigated DNA damage using human cultured cell lines and 32P-labeled DNA fragments obtained from the human p53 and p16 tumor suppressor genes and the c-Ha-ras-1 proto-oncogene. Catechol increased the amount of 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxodG), which is known to be correlated with the incidence of cancer, in a human leukemia cell line HL-60, whereas the amount of 8-oxodG in its hydrogen peroxide (H2O2)-resistant clone HP100 was not increased. The formation of 8-oxodG in calf thymus DNA was increased by catechol in the presence of Cu(2+). Catechol caused damage to 32P-labeled DNA fragments in the presence of Cu(2+). When NADH was added, DNA damage was markedly enhanced and clearly observed at relatively low concentrations of catechol (<1 microM). DNA cleavage was enhanced by piperidine treatment, suggesting that catechol plus NADH caused not only deoxyribose phosphate backbone breakage but also base modification. Catechol plus NADH frequently modified thymine residues. Bathocuproine, a specific Cu(+) chelator and catalase inhibited the DNA damage, indicating the participation of Cu(+) and H2O2 in DNA damage. Typical hydroxyl radical scavengers did not inhibit catechol plus Cu(2+)-induced DNA damage, whereas methional completely inhibited it. These results suggest that reactive species derived from the reaction of H2O2 with Cu(+) participates in catechol-induced DNA damage. Therefore, /the authors/ conclude that oxidative DNA damage by catechol through the generation of H2O2 plays an important role in the carcinogenic process of catechol and benzene.
PMID:11470755 Oikawa S et al; Carcinogenesis 22 (8): 1239-45 (2001)
We examined the redox properties of the "carcinogenic" catechol and the "noncarcinogenic" hydroquinone in relation to different DNA damaging activities and carcinogenicity using 32P-labeled DNA fragments obtained from the human genes. In the presence of endogenous NADH and Cu2+, catechol induces stronger DNA damage than hydroquinone, although the magnitudes of their DNA damaging activities were reversed in the absence of NADH. In both cases, DNA damage resulted from base modification at guanine and thymine residues in addition to strand breakage induced by Cu+ and H2O2, generated during the oxidation of catechol and hydroquinone into 1,2-benzoquinone and 1,4-benzoquinone, respectively. EPR and 1H NMR studies indicated that 1,2-benzoquinone is converted directly into catechol through a nonenzymatic two-electron reduction by NADH whereas 1,4-benzoquinone is reduced into hydroquinone through a semiquinone radical intermediate through two cycles of one-electron reduction. The reduction of 1,2-benzoquinone by NADH proceeds more rapidly than that of 1,4-benzoquinone. This study demonstrates that the rapid 1,2-benzoquinone two-electron reduction accelerates the redox reaction turnover between catechol and 1,2-benzoquinone, resulting in the enhancement of DNA damage. These results suggest that the differences in NADH-mediated redox properties of catechol and hydroquinone contribute to their different carcinogenicities
PMID:11800599 Hirakawa K et al; Chem Res Toxicol 15 (1): 76-82 (2002)
Catechol is possibly carcinogenic to humans (International Agency for Research on Cancer, IARC). The key mechanism could include its oxidative DNA-damaging effect in combination with reductive-oxidative metals like Cu. We found that DNA damage was suppressed by introducing an alpha-carbonyl group to catechol at C4-position to produce carbonyl catechols. During the oxidative DNA-damaging process, catechols but not carbonyl catechols were oxidized to o-quinone; however, coexisting Cu(II) was reduced to Cu(I). Carbonyl catechols were possibly arrested at the oxidation step of semiquinones in the presence of Cu(II). Cu(I)-binding to DNA was stronger than Cu(II)-binding, on the basis of the circular dichroism spectral change. None of the carbonyl catechols induced such change, suggesting sequestration of Cu(I) from DNA. Solid-phase extraction experiments and spectrophotometric analyses showed the formation of semiquinone chelates with Cu(I). Thus, chelate formation could explain the suppression mechanism of the Cu-catechol-dependent DNA damage by terminating the reduction-oxidation cycle. Structural modifications such as introducing an alpha-carbonyl group to catechol at C4-position would contribute to reducing the risk and improving industrial and medical potentials of aromatic/phenolic compounds ... .
PMID:20832456 Ando M et al; Toxicol Lett. 199 (3): 213-7 (2010)