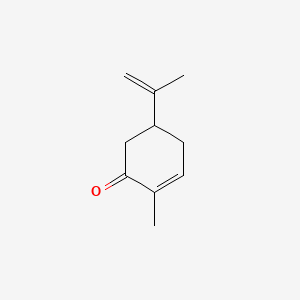

1. (rs)-5-isopropenyl-2-methylcyclohex-2-en-1-one
2. 2-methyl-5-(1-methylethenyl)-2-cyclohexene-1-one
3. 2-methyl-5-isopropenyl-2-cyclohexenone
4. 5-isopropyl-2-methyl-2-cyclohexen-1-one
5. Carvone, (+--)-
6. Carvone, (r)-isomer
7. Carvone, (s)-isomer
8. Limonen-6-one
1. 99-49-0
2. 2-methyl-5-(prop-1-en-2-yl)cyclohex-2-enone
3. Karvon
4. Dl-carvone
5. 1-carvone
6. P-mentha-6,8-dien-2-one
7. 2-methyl-5-isopropenyl-2-cyclohexenone
8. Carvone [iso]
9. D-cavone
10. 2-methyl-5-prop-1-en-2-ylcyclohex-2-en-1-one
11. 2-cyclohexen-1-one, 2-methyl-5-(1-methylethenyl)-
12. 6,8(9)-p-menthadien-2-one
13. 2-methyl-5-(1-methylethenyl)-2-cyclohexen-1-one
14. Nci-c55867
15. (+/-)-carvone
16. 6,8-p-menthadien-2-one
17. 2-methyl-5-(prop-1-en-2-yl)cyclohex-2-en-1-one
18. 75gk9xia8i
19. P-mentha-6,8-dien-2-one, (r)-(-)-
20. 2-cyclohexen-1-one, 2-methyl-5-(1-methylethenyl)-, (r)-
21. Chebi:38265
22. P-mentha-1(6),8-dien-2-one
23. Nsc6275
24. Nsc-6275
25. 2-methyl-5-(1-methylethenyl)-2-cyclohexene-1-one
26. Mfcd00062996
27. Carvone 100 Microg/ml In Acetonitrile
28. Carvone (natural)
29. L-6,8(9)-p-menthadien-2-one
30. Fema Number 2249
31. 6,8-p-menthadien-2-on
32. Limonen-6-one
33. D-p-mentha-1(6),8-dien-2-one
34. Fema No. 2249
35. Hsdb 707
36. (+-)-carvone
37. Nsc 6275
38. Einecs 202-759-5
39. Unii-75gk9xia8i
40. Delta(sup 6,8)-(9)-terpadienone-2
41. Brn 1364206
42. Carvon
43. A Carvone
44. Ai3-08877
45. Mfcd00001578
46. Mfcd00062997
47. D-p-mentha-6,8,(9)-dien-2-one
48. .alpha.-carvone
49. Delta-1-methyl-4-isopropenyl-6-cyclohexen-2-one
50. 5-isopropenyl-2-methylcyclohex-2-en-1-one
51. Carvone [hsdb]
52. Carvone [inci]
53. Carvone [mi]
54. Carvone, Dl-
55. 5-isopropenyl-2-methyl-cyclohex-2-en-1-one
56. 2-methyl-5-(1-propen-2-yl)-2-cyclohexenone
57. Dsstox_cid_27426
58. Dsstox_rid_82339
59. Nciopen2_001348
60. (rs)-5-isopropenyl-2-methylcyclohex-2-en-1-one
61. Dsstox_gsid_47426
62. Schembl39408
63. 4-07-00-00316 (beilstein Handbook Reference)
64. Carvone Dl-form [mi]
65. Chembl15676
66. Carvone, (+-)-
67. Carvone, (+/-)-
68. Dtxsid8047426
69. Amy4152
70. Hms1789n08
71. Nsc93738
72. Tox21_302547
73. Bbl010103
74. Nsc-93738
75. Stk801456
76. Akos000121377
77. Akos016843655
78. Cas-99-49-0
79. Ncgc00256915-01
80. Wln: L6v Butj B1 Ey1 & U1
81. .delta.(sup 6,8)-(9)-terpadienone-2
82. As-10471
83. Nci60_008753
84. Sy010704
85. Sy012922
86. Sy274718
87. 2-methyl-5-isopropenyl-2-cyclohexen-1-one
88. Db-054736
89. Cs-0033814
90. Ft-0600385
91. Ft-0605067
92. Ft-0658046
93. Fema No. 2249, (+/-)-
94. O10834
95. (-)-2-methyl-5-isopropenyl-2-cyclohexen-1-one
96. A858458
97. Q416800
98. .delta.-1-methyl-4-isopropenyl-6-cyclohexen-2-one
99. 5-isopropenyl-2-methyl-2-cyclohexen-1-one, (r)-
100. W-100036
101. 2-methyl-5-(1-methyl-1-ethenyl)-2-cyclohexen-1-one
| Molecular Weight | 150.22 g/mol |
|---|---|
| Molecular Formula | C10H14O |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 150.104465066 g/mol |
| Monoisotopic Mass | 150.104465066 g/mol |
| Topological Polar Surface Area | 17.1 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 223 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Veterinary use: Carvi aetheroleum (containing D-carvone) is used in veterinary medicinal products to facilitate breathing in new-borne animals, and to treat flatulence and disturbances of the stomach and the gut in farmed animals.
EFSA Journal 12 (7): 3806 (2014)
...Carvi aetheroleum and carvi fructus are registered as herbal medicinal products by the European Medicines Agency. Uses as a laxative, in colic treatment, as a breath freshener, or to help digestion in young children have been reported. Other properties claimed for caraway seeds include antispasmodic, carminative, emmenagogue, expectorant, galactagogue, stimulant, stomachic, and tonic properties.
EFSA Journal 12 (7): 3806 (2014)
Antineoplastic Agents, Phytogenic
Agents obtained from higher plants that have demonstrable cytostatic or antineoplastic activity. (See all compounds classified as Antineoplastic Agents, Phytogenic.)
In humans, D-carvone pharmacokinetics was investigated in 15 male volunteers who, after a 10 hr fast, took 5 capsules of an immediate release formulation containing 20 mg caraway oil. Carvone concentrations in plasma were determined by GC/MS, with a limit of quantification of 0.5 ng/mL for carvone. Pharmacokinetic parameters were determined, i.e., area-under the plasma-concentration curve (AUC) of 28.9+/- 20.0 ng.mL/hr , plasma peak concentration (Cmax) of 14.8+/-10.4 ng/mL with a time to reach Cmax (Tmax) of 1.3 hours and a half life of 2.4 hours. Inter-individual differences determined as the coefficients of variation in AUC, Cmax, and t1/2 were 69%, 74%, and 50% respectively. /D-Carvone/
EFSA Journal 12 (7): 3806 (2014)
As part of a program aiming at the selection of yeast strains which might be of interest as sources of natural flavors and fragrances, the bioreduction of (4R)-(-)-carvone and (1R)-(-)-myrtenal by whole-cells of non-conventional yeasts (NCYs) belonging to the genera Candida, Cryptococcus, Debaryomyces, Hanseniaspora, Kazachstania, Kluyveromyces, Lindnera, Nakaseomyces, Vanderwaltozyma, and Wickerhamomyces was studied. Volatiles produced were sampled by means of headspace solid-phase microextraction (SPME) and the compounds were analyzed and identified by gas chromatography-mass spectroscopy (GC-MS). Yields (expressed as % of biotransformation) varied in dependence of the strain. The reduction of both (4R)-(-)-carvone and (1R)-(-)-myrtenal were catalyzed by some ene-reductases (ERs) and/or carbonyl reductases (CRs), which determined the formation of (1R,4R)-dihydrocarvone and (1R)-myrtenol respectively, as main flavoring products. The potential of NCYs as novel whole-cell biocatalysts for selective biotransformation of electron-poor alkenes for producing flavors and fragrances of industrial interest is discussed.
PMID:23681058 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6270020 Goretti M et al; Molecules 18 (5): 5736-48 (2013)
... Ketones (e.g., carvone and menthone) are reduced to secondary alcohols which are then excreted as glucuronides.
Parke, D. V. The Biochemistry of Foreign Compounds. Oxford: Pergamon Press, 1968., p. 149
The cyclic monoterpene ketone (-)-carvone was metabolized by the plant pathogenic fungus Absidia glauca. After 4 days of incubation, the diol 10-hydroxy-(+)-neodihydrocarveol was formed. The absolute configuration and structure of the crystalline substance was identified by means of X-ray diffraction and by spectroscopic techniques (MS, IR, and NMR). The antimicrobial activity of the substrate and metabolite was assayed with human pathogenic microorganisms.
PMID:18998407 Demirci F et al; Z Naturforsch C 59 (5-6): 389-92 (2004)
D-Carvone toxicokinetics in humans features rapid elimination with a half life of 2.4 hours, no data are available for L-carvone. No toxicokinetic data on carvone in animals are available. The evidence from in vivo, in vitro, and in silico assessments has shown that carvone metabolism is likely to be different in humans and rats - with further possible differences between metabolism in male and female rats. It is also evident that when compared with carvone itself, the metabolites are not likely to be different in terms of GI uptake or half-life in the body. Toxicokinetic data on other monoterpenes in the rat such as menthol suggest that metabolism involves conjugation to a glucuronide for which enterohepatic recirculation occurs in the rat but not in humans. Considering the molecular weight of glucuronidated carvone metabolites, they may undergo enterohepatic recirculation in rats but not in humans, making the rat more sensitive than humans for these compounds. /D-Carvone and L-Carvone/
EFSA Journal 12 (7): 3806 (2014)
In vivo metabolism of D- and L-carvone has been investigated in six human volunteers (three males, three females) after oral dosing (1 mg/kg bw), with collection of urine samples 24 hr before and after the ingestion of each enantiomer separately. Chemical structures of the metabolites were elucidated using mass spectral analysis in combination with metabolite syntheses and NMR analysis. For this, the urinary samples were treated with sulphatase and glucuronidase, assuming conjugation of phase I metabolites. However, no quantitative data on excretion of conjugated forms of the metabolites were reported. The study identified three side-chain oxidation products as the main primary unconjugated metabolites of D- and L-carvone: dihydrocarvonic acid, carvonic acid, and uroterpenolone, with 10-hydroxycarvone as the proposed intermediate metabolic step. However, unlike other species, the presence of 10-hydroxycarvone was not detected in humans and /it was/ suggested this was due to more efficient oxidation of 10-hydroxycarvone leading to carvonic acid. According to /the study/, there were no differences in the metabolism of D- and L-carvone. However, the results presented only refer to "after carvone ingestion", although apparently both carvone enantiomers were ingested by the volunteers in independent trials. According to the author, all metabolites were identical after the application of either D- or L-carvone. However, the configurations of metabolites were not identified and the chromatographic analyses were only performed on a nonchiral stationary phase. This experimental set-up does not allow differentiation of the stereospecific metabolism of D- and L-carvone. /D-Carvone and L-Carvone/
EFSA Journal 12 (7): 3806 (2014)
D-Carvone toxicokinetics in humans features rapid elimination with a half life of 2.4 hours, no data are available for L-carvone. /D-Carvone/
EFSA Journal 12 (7): 3806 (2014)