

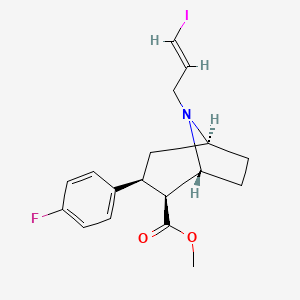

1. 2-carbomethoxy-3-(4-fluorophenyl)-n-(1-iodoprop-1-en-3-yl)nortropane
2. Iacft
3. N-iodoallyl-2-carbomethoxy-3-(4-fluorophenyl)tropane
1. 180468-34-2
2. 1q4092099o
3. 8-azabicyclo(3.2.1)octane-2-carboxylic Acid, 3-(4-fluorophenyl)-8-((2e)-3-iodo-2-propenyl)-, Methyl Ester, (1r,2s,3s,5s)-
4. Iacft
5. Methyl (1r,2s,3s,5s)-3-(4-fluorophenyl)-8-[(e)-3-iodoprop-2-enyl]-8-azabicyclo[3.2.1]octane-2-carboxylate
6. Unii-1q4092099o
7. Iacft [mi]
8. N-iodoallyl-2-carbomethoxy-3-(4-fluorophenyl)tropane
9. Schembl1650069
10. Chembl4301492
11. Chebi:135696
12. Dtxsid901027571
13. 2beta-carbomethoxy-3beta-(4-fluorophenyl)-n-(3-iodo-e-allyl)nortropane
14. Db04947
15. Q4737006
16. (1r,5s)-3beta-(4-fluorophenyl)-8-(3-iodoallyl)-8-demethyltropane-2beta-carboxylic Acid Methyl Ester
17. (2r)-1-[(e)-3-iodoallyl]-2alpha,6alpha-ethano-3beta-(methoxycarbonyl)-4beta-(4-fluorophenyl)piperidine
18. 8-azabicyclo(3.2.1)octane-2-carboxylic Acid, 3-(4-fluorophenyl)-8-((2e)-3-iodo-2-propen-1-yl)-, Methyl Ester, (1r,2s,3s,5s)-
19. 8-azabicyclo(3.2.1)octane-2-carboxylic Acid, 3-(4-fluorophenyl)-8-(3-iodo-2-propenyl)-, Methyl Ester, (1r-(1.alpha.,2.alpha.,3.alpha.,5.alpha.,8(e)))-
20. 8-azabicyclo(3.2.1)octane-2-carboxylic Acid, 3-(4-fluorophenyl)-8-(3-iodo-2-propenyl)-, Methyl Ester, (1r-(1alpha,2alpha,3alpha,5alpha,8(e)))-
| Molecular Weight | 429.3 g/mol |
|---|---|
| Molecular Formula | C18H21FINO2 |
| XLogP3 | 3.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Exact Mass | 429.06010 g/mol |
| Monoisotopic Mass | 429.06010 g/mol |
| Topological Polar Surface Area | 29.5 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 450 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in attention deficit/hyperactivity disorder (ADHD), parkinson's disease, and pediatric indications.
Altropane is a molecular-imaging agent that specifically binds to the dopamine transporter (DAT) protein found on the surface of dopamine-producing neurons, making it visible during SPECT imaging. Since most forms of Parkinsonian Syndromes result in a decreased number of dopamine-producing cells, it would be expected that these patients also have fewer DATs than do patients without PS. Thus, it is believed that altropane used in conjunction with SPECT imaging could be a useful test to distinguish Parkinsonian Syndrome tremors from non-Parkinsonian tremor: non-Parkinsonian patients would have more altropane-binding visible in the SPECT image, while Parkinsonian patients would have less.
Positron emission tomography (PET) cameras are expensive and scarce, and the tests are non-reimbursable. A less costly and more available test such as a single photon emission computed tomography (SPECT) may be helpful in the diagnosis of early or atypical Parkinson's disease (PD) if its sensitivity is comparable to a PET scan. Altropane is an iodinated form of the N-allyl analog of WIN 35,428 which acts as a dopamine transport inhibitor. When radiolabeled with the gamma emitting isotope [123I], altropane serves as a SPECT ligand with high affinity and selectivity for the dopamine transporter. It is a good marker for dopamine neurons and is useful in detecting PD.