

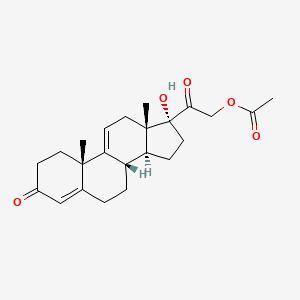

1. 4,9(11)-pregnadien-17alpha,21-diol-3,20-dione-21-acetate
1. 7753-60-8
2. Retaane
3. Al 3789
4. Al-3789
5. Anecortave Acetate [usan]
6. Nsc 15475
7. Nsc 24345
8. Anecortave [inn]
9. Nsc-15475
10. Nsc-24345
11. 17,21-dihydroxypregna-4,9(11)-diene-3,20-dione 21-acetate
12. Y0pc411k4t
13. 21-(acetyloxy)-17-hydroxypregna-4,9(11)-diene-3,20-dione
14. [2-[(8s,10s,13s,14s,17r)-17-hydroxy-10,13-dimethyl-3-oxo-2,6,7,8,12,14,15,16-octahydro-1h-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl] Acetate
15. 17-hydroxy-3,20-dioxopregna-4,9(11)-dien-21-yl Acetate
16. Anecortave Acetate (200 Mg)f0e2980.997mg/mg(ai)
17. Retaane Suspension
18. Unii-y0pc411k4t
19. Anecortave-acetate
20. Ncgc00181018-01
21. Einecs 231-812-5
22. Anecortave [mart.]
23. Dsstox_cid_26805
24. Dsstox_rid_81919
25. Dsstox_gsid_46805
26. Schembl94110
27. 17-hydroxy-3,20-dioxopregna-4,9(11)-dien-21-yl Acetate #
28. Anecortave Acetate [mi]
29. Anecortave Acetate (jan/usan)
30. Anecortave Acetate [jan]
31. Chembl2106613
32. Dtxsid5046805
33. Chebi:31215
34. Ex-a5253
35. Nsc15475
36. Nsc24345
37. Zinc3931050
38. Tox21_112668
39. 17-alpha,21-dihydroxypregna-4,9(11)-diene-3,20-dione 21-acetate
40. Akos015917580
41. Db05288
42. Cas-7753-60-8
43. Hy-116868
44. Cs-0066714
45. D01733
46. Q4761567
47. Hydrocortisone Acetate Impurity E [ep Impurity]
48. 21-acetoxy-17-hydroxy-4,9(11)-pregnadiene-3,20-dione
49. 17alpha-hydroxy-21-acetoxypregna-4,9(11)-diene-3,20-dione
50. 4,9(11)-pregnadien-17alpha,21-diol-3,20-dione-21-acetate
51. Pregn-4,9(11)-dien-17,21-diol-3,20-dione, Acetate(ester)
52. 17alpha-hydroxy-21 -acetoxy-pregna-4,9(11)-diene-3,20-dione
53. Pregna-4,9(11)-diene-3,20-dione, 21-(acetyloxy)-17-hydroxy-
| Molecular Weight | 386.5 g/mol |
|---|---|
| Molecular Formula | C23H30O5 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 386.20932405 g/mol |
| Monoisotopic Mass | 386.20932405 g/mol |
| Topological Polar Surface Area | 80.7 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 808 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in glaucoma and macular degeneration.
Anecortave acetate functions as an antiangiogenic agent, inhibiting blood vessel growth by decreasing extracellular protease expression and inhibiting endothelial cell migration. Its angiostatic activity does not seem to be mediated through any of the commonly known pharmacological receptors. (Ophthalmology 2004;111:2316-7) RETAANE blocks signals from multiple growth factors because it acts downstream and independent of the initiating angiogenic stimuli and inhibits angiogenesis subsequent to the angiogenic stimulation.