



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports

1. 48933, Cgp
2. Cgp 48933
3. Diovan
4. Kalpress
5. Miten
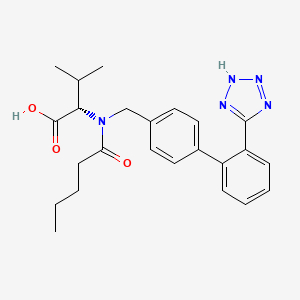
6. N-valeryl-n-((2'-(1h-tetrazol-5-yl)biphenyl-4-yl)methyl)valine
7. Nisis
8. Provas
9. Tareg
10. Vals
1. 137862-53-4
2. Diovan
3. Tareg
4. Cgp 48933
5. Provas
6. Exforge
7. Cgp-48933
8. (s)-2-(n-((2'-(1h-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)pentanamido)-3-methylbutanoic Acid
9. 137863-60-6
10. Prexxartan
11. Mfcd00865840
12. N-(p-(o-1h-tetrazol-5-ylphenyl)benzyl)-n-valeryl-l-valine
13. (2s)-3-methyl-2-[pentanoyl-[[4-[2-(2h-tetrazol-5-yl)phenyl]phenyl]methyl]amino]butanoic Acid
14. Nsc-758927
15. Chembl1069
16. L-valine, N-(1-oxopentyl)-n-[[2'-(2h-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-
17. 80m03yxj7i
18. Chebi:9927
19. (s)-2-(n-((2'-(1h-tetrazol-5-yl)biphenyl-4-yl)methyl)pentanamido)-3-methylbutanoic Acid
20. L-valine, N-(1-oxopentyl)-n-((2'-(1h-tetrazol-5-yl)(1,1'-biphenyl)-4-yl)methyl)-
21. Kalpress
22. Miten
23. Nisis
24. Vals
25. (s)-n-valeryl-n-{[2'-(1h-tetrazol-5-yl)biphenyl-4-yl]-methyl}-valine
26. N-(1-oxopentyl)-n-[[2'-(1h-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-l-valine
27. Valzaar
28. Valtan
29. [3h]valsartan
30. (2~{s})-3-methyl-2-[pentanoyl-[[4-[2-(2~{h}-1,2,3,4-tetrazol-5-yl)phenyl]phenyl]methyl]amino]butanoic Acid
31. Smr000466318
32. Diovan (tn)
33. Unii-80m03yxj7i
34. Valpression
35. Valsartana
36. Varexan
37. Hsdb 7519
38. Valsartan [usan:usp:inn:ban]
39. Valsartan,(s)
40. Valsartan- Bio-x
41. (2s)-3-methyl-2-(pentanoyl-((4-(2-(2h-tetrazol-5-yl)phenyl)phenyl)methyl)amino)butanoic Acid
42. (2s)-3-methyl-2-[pentanoyl-[[4-[2-(1h-tetrazol-5-yl)phenyl]phenyl]methyl]amino]butanoic Acid
43. Valsartan (diovan)
44. Spectrum_001796
45. Valsartan [inn]
46. Valsartan [jan]
47. Valsartan [mi]
48. Valsartan [hsdb]
49. Valsartan [usan]
50. Spectrum2_001120
51. Spectrum3_001831
52. Spectrum4_000749
53. Spectrum5_001582
54. Valsartan [vandf]
55. Prexxartan (oral Solution)
56. Valsartan [mart.]
57. Ec 604-045-2
58. Schembl2542
59. Valsartan [usp-rs]
60. Valsartan [who-dd]
61. Bspbio_003501
62. Gtpl593
63. Kbiogr_001078
64. Kbioss_002289
65. Valsartan [ema Epar]
66. Mls000759423
67. Mls001424088
68. Bidd:gt0345
69. Spectrum1505209
70. Spbio_001260
71. Valsartan (jp17/usp/inn)
72. Gtpl3937
73. Valsartan [ep Impurity]
74. Valsartan [orange Book]
75. Dtxsid6023735
76. Valsartan [ep Monograph]
77. Valsartan, >=98% (hplc)
78. Dafiro Component Valsartan
79. Kbio2_002287
80. Kbio2_004855
81. Kbio2_007423
82. Kbio3_003006
83. Ezr-104
84. Val-489
85. Valsartan [usp Monograph]
86. Copalia Component Valsartan
87. Exforge Component Valsartan
88. Imprida Component Valsartan
89. Hms1922l21
90. Hms2051l12
91. Hms2093k22
92. Hms2232f05
93. Hms3651e04
94. Hms3715p12
95. Pharmakon1600-01505209
96. Entresto Component Valsartan
97. Valturna Component Valsartan
98. (2r)-3-methyl-2-[pentanoyl[[2'-(1h-tetrazol-5-yl)biphenyl-4-yl]methyl]amino]butanoic Acid (valsartan (r)-enantiomer)
99. Act02671
100. Bcp05184
101. Zinc3875259
102. Bdbm50049186
103. Nsc758927
104. S1894
105. Dafiro-hct Component Valsartan
106. Diovan Hct Component Valsartan
107. Valsartan Component Of Copalia
108. Valsartan Component Of Exforge
109. Akos015914315
110. Akos015994698
111. Byvalson Component Of Valsartan
112. Copalia-hct Component Valsartan
113. Exforge Hct Component Valsartan
114. Imprida-hct Component Valsartan
115. Valsartan Component Of Byvalson
116. Valsartan Component Of Entresto
117. Valsartan Component Of Immprida
118. Valsartan Component Of Valturna
119. Ac-4543
120. Am90287
121. Ccg-101028
122. Ccg-221275
123. Cs-1967
124. Db00177
125. Ds-1248
126. Ks-1194
127. Nc00278
128. Nsc 758927
129. N-(1-oxopentyl)-n-[[2'-(2h-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-l-valine
130. N-pentanoyl-n-{[2'-(1h-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl}-l-valine
131. Ncgc00178027-08
132. Ncgc00178027-11
133. Valsartan Component Of Dafiro-hct
134. Valsartan Component Of Diovan Hct
135. Bv164506
136. Hy-18204
137. Valsartan Component Of Copalia-hct
138. Valsartan Component Of Exforge Hct
139. Valsartan Component Of Imprida-hct
140. Sbi-0206738.p001
141. Sw197658-2
142. V-120
143. V0112
144. 62v534
145. D00400
146. Ab00639940-06
147. Ab00639940-08
148. Ab00639940_09
149. Ab00639940_11
150. Q155472
151. Sr-05000001928
152. J-007068
153. Sr-05000001928-1
154. Brd-k45158365-001-02-3
155. Brd-k45158365-001-05-6
156. Valsartan, European Pharmacopoeia (ep) Reference Standard
157. N-valeryl-n-[2'-(5-tetrazolyl)biphenyl-4-ylmethyl]-l-valine
158. Valsartan, United States Pharmacopeia (usp) Reference Standard
159. N-valeryl-n-[2'-(1h-tetrazol-5-yl)biphenyl-4-ylmethyl]-l-valine
160. Valsartan, Pharmaceutical Secondary Standard; Certified Reference Material
161. (5)-2-{n-[(2'-(1h-tetrazol-5-yl)biphenyl-4-yl)methyl]pentanamido}-3-methylbutanoic Acid
162. (s)-3-methyl-2-{pentanoyl-[2'-(1h-tetrazol-5-yl)-biphenyl-4-ylmethyl]-amino}-butyric Acid
163. 3-methyl-2-{pentanoyl-[2''-(1h-tetrazol-5-yl)-biphenyl-4-yl]-amino}-butyric Acid
164. L-valine,n-(1-oxopentyl)-n-[[2'-(1h-tetrazol-5-yl)[1,1'-biphenyl]-3-yl]methyl]- (9ci)
165. L-valine,n-(1-oxopentyl)-n-[[2'-(1h-tetrazol-5-yl)[1,1'-biphenyl]-3-yl]methyl]-(9ci)
166. N-(1-oxopentyl)-n-[[2'-(1h-tetrazol-5-yl) [1,1'-bi-phenyl]-4-yl]methyl]-l-valine
167. N-(1-oxopentyl)-n-[[2'-(1h-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-(l)-valine
168. N-pentanoyl-n-{[2'-(1h-tetrazol-5-yl)-1,1'-biphenyl-4-yl]methyl}-l-valine
169. Valsartan For Peak Identification, European Pharmacopoeia (ep) Reference Standard
170. Valsartan For System Suitability, European Pharmacopoeia (ep) Reference Standard
171. (2~{s})-3-methyl-2-[pentanoyl-[[4-[2-(2~{h}-1,2,3,4-tetrazol-5-yl)phenyl]phenyl]methyl]amino]butanoic Acid; Diovan; Tareg; Provas; Exforge; N-(p-(o-1h-tetrazol-5-ylphenyl)benzyl)-n-valeryl-l-valine
172. (2s)-3-methyl-2-[n-({4-[2-(2h-1,2,3,4-tetrazol-5-yl)phenyl]phenyl}methyl)pentanamido]butanoic Acid
173. (2s)-3-methyl-2-[pentanoyl-[[4-[2-(2h-tetrazol-5-yl)phenyl]phenyl]methyl]amino]butanoic Acid.
174. (2s)-3-methyl-2-{n-pentanoyl-n'-[2'-(1h-tetrazol-5-yl)-biphenyl-4-ylmethyl]-amino}-butanoic Acid
175. (2s)-3-methyl-2-{pentanoyl-[2'-(1h-tetrazol-5-yl)-biphenyl-4-ylmethyl]-amino}-butyric Acid
176. (s)-2-(n-((2'-(1h-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)pentanamido)-3-methylbutanoicacid
177. (s)-2-(n-((2'-(2h-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)pentanamido)-3-methylbutanoic Acid
178. (s)-3-methyl-2-[n-({4-[2-(2h-1,2,3,4-tetrazol-5-yl) Phenyl]phenyl}methyl)pentanamido]butanoic Acid
179. (s)-3-methyl-2-[n-({4-[2-(2h-1,2,3,4-tetrazol-5-yl)phenyl]phenyl}methyl)pentanamido]butanoic Acid
180. (s)-3-methyl-2-{pentanoyl-[-2'-(1h-tetrazol-5-yl)-biphenyl-4-ylmethyl]-amino}-butyric Acid
181. (s)-3-methyl-2-{pentanoyl-[2''-(1h-tetrazol-5-yl)-biphenyl-4-ylmethyl]-amino}-butyric Acid
182. (s)-3-methyl-2-{pentanoyl-[2''-(2h-tetrazol-5-yl)-biphenyl-4-ylmethyl]-amino}-butyric Acid
183. (s)-n-(1-carboxy-2-methyl-prop-1-yl)-n-pentanoyl-n-[2'(1h-tetrazol-5-yl)biphenyl-4-yl-methyl]amine
184. (s)-n-(1-carboxy-2-methyl-prop-1-yl)-n-pentanoyl-n-[2'-(1h-tetrazol-5-yl)-biphenyl-4-ylmethyl]-amine
185. (s)-n-(1-carboxy-2-methyl-prop-1-yl)-n-pentanoyl-n-[2'-(1h-tetrazol-5-yl)biphenyl-4-ylmethyl]-amine
186. 2-{[2''-(2,3-dihydro-1h-tetrazol-5-yl)-biphenyl-4-ylmethyl]-pentanoyl-amino}-3-methyl-butyric Acid
187. 3-methyl-2-{((s)-pentanoyl)-[2''-(1h-tetrazol-5-yl)-biphenyl-4-ylmethyl]-amino}-butyric Acid
188. U35
| Molecular Weight | 435.5 g/mol |
|---|---|
| Molecular Formula | C24H29N5O3 |
| XLogP3 | 4.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 10 |
| Exact Mass | 435.22703980 g/mol |
| Monoisotopic Mass | 435.22703980 g/mol |
| Topological Polar Surface Area | 112 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 608 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Diovan |
| PubMed Health | Valsartan (By mouth) |
| Drug Classes | Cardiovascular Agent, Renal Protective Agent |
| Drug Label | Diovan (valsartan) is a nonpeptide, orally active, and specific angiotensin II receptor blocker acting on the AT1 receptor subtype.Valsartan is chemically described as N-(1-oxopentyl)-N-[[2-(1H-tetrazol-5-yl) [1,1-biphenyl]-4-yl]methyl]-L-valin... |
| Active Ingredient | Valsartan |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 160mg; 80mg; 40mg; 320mg |
| Market Status | Prescription |
| Company | Novartis |
| 2 of 4 | |
|---|---|
| Drug Name | Valsartan |
| PubMed Health | Valsartan (By mouth) |
| Drug Classes | Cardiovascular Agent, Renal Protective Agent |
| Drug Label | Diovan (valsartan) is a nonpeptide, orally active, and specific angiotensin II receptor blocker acting on the AT1 receptor subtype.Valsartan is chemically described as N-(1-oxopentyl)-N-[[2-(1H-tetrazol-5-yl) [1,1-biphenyl]-4-yl]methyl]-L-valin... |
| Active Ingredient | Valsartan |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 160mg; 320mg; 80mg; 40mg |
| Market Status | Tentative Approval; Prescription |
| Company | Ohm Labs; Ivax Pharms; Alembic Pharms; Mylan Labs; Aurobindo Pharma; Lupin; Watson Labs; Dr Reddys Labs |
| 3 of 4 | |
|---|---|
| Drug Name | Diovan |
| PubMed Health | Valsartan (By mouth) |
| Drug Classes | Cardiovascular Agent, Renal Protective Agent |
| Drug Label | Diovan (valsartan) is a nonpeptide, orally active, and specific angiotensin II receptor blocker acting on the AT1 receptor subtype.Valsartan is chemically described as N-(1-oxopentyl)-N-[[2-(1H-tetrazol-5-yl) [1,1-biphenyl]-4-yl]methyl]-L-valin... |
| Active Ingredient | Valsartan |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 160mg; 80mg; 40mg; 320mg |
| Market Status | Prescription |
| Company | Novartis |
| 4 of 4 | |
|---|---|
| Drug Name | Valsartan |
| PubMed Health | Valsartan (By mouth) |
| Drug Classes | Cardiovascular Agent, Renal Protective Agent |
| Drug Label | Diovan (valsartan) is a nonpeptide, orally active, and specific angiotensin II receptor blocker acting on the AT1 receptor subtype.Valsartan is chemically described as N-(1-oxopentyl)-N-[[2-(1H-tetrazol-5-yl) [1,1-biphenyl]-4-yl]methyl]-L-valin... |
| Active Ingredient | Valsartan |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 160mg; 320mg; 80mg; 40mg |
| Market Status | Tentative Approval; Prescription |
| Company | Ohm Labs; Ivax Pharms; Alembic Pharms; Mylan Labs; Aurobindo Pharma; Lupin; Watson Labs; Dr Reddys Labs |
Angiotensin II Type 1 Receptor Blockers; Antihypertensive Agents
NIH; DailyMed. Current Medication Information for Diovan (Valsartan) Tablet (Revised: March 2014). Available from, as of May 28, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Diovan is an angiotensin II receptor blocker (ARB) indicated for: treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. /Included in US product labeling/
NIH; DailyMed. Current Medication Information for Diovan (Valsartan) Tablet (Revised: March 2014). Available from, as of May 28, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5ddba454-f3e6-43c2-a7a6-58365d297213
Diovan is an angiotensin II receptor blocker (ARB) indicated for: reduction of cardiovascular mortality in clinically stable patients with left ventricular failure or left ventricular dysfunction following myocardial infarction. /Included in US product labeling/
NIH; DailyMed. Current Medication Information for Diovan (Valsartan) Tablet (Revised: March 2014). Available from, as of May 28, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5ddba454-f3e6-43c2-a7a6-58365d297213
Diovan is an angiotensin II receptor blocker (ARB) indicated for: treatment of heart failure (NYHA class II-IV); Diovan significantly reduced hospitalization for heart failure. /Included in US product labeling/
NIH; DailyMed. Current Medication Information for Diovan (Valsartan) Tablet (Revised: March 2014). Available from, as of May 28, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5ddba454-f3e6-43c2-a7a6-58365d297213
For more Therapeutic Uses (Complete) data for VALSARTAN (6 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: FETAL TOXICITY. When pregnancy is detected, discontinue Diovan as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus.
NIH; DailyMed. Current Medication Information for Diovan (Valsartan) Tablet (Revised: March 2014). Available from, as of May 28, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5ddba454-f3e6-43c2-a7a6-58365d297213
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Diovan as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimesters of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus. In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue Diovan, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in utero exposure to Diovan for hypotension, oliguria, and hyperkalemia.
NIH; DailyMed. Current Medication Information for Diovan (Valsartan) Tablet (Revised: March 2014). Available from, as of May 28, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5ddba454-f3e6-43c2-a7a6-58365d297213
Angiotensin II (A-II) is the main effector of the renin-angiotensin system. A-II functions by binding its type 1 (AT1) receptors to cause vasoconstriction and retention of sodium and fluid. Several AT1 receptor antagonists-a group of drugs collectively called "sartans"-have been marketed during the past few years for treatment of hypertension and heart failure. At least 15 case reports describe oligohydramnios, fetal growth retardation, pulmonary hypoplasia, limb contractures, and calvarial hypoplasia in various combinations in association with maternal losartan, candesartan, valsartan, or telmisartan treatment during the second or third trimester of pregnancy. Stillbirth or neonatal death is frequent in these reports, and surviving infants may exhibit renal damage. The fetal abnormalities, which are strikingly similar to those produced by maternal treatment with angiotensin-converting enzyme (ACE) inhibitors during the second and third trimesters of pregnancy, are probably related to extreme sensitivity of the fetus to the hypotensive action of these drugs. ...
PMID:15669052 Alwan S et al; Birth Defects Res A Clin Mol Teratol 73 (2): 123-30 (2005)
Valsartan is distributed into milk in rats. It is not known whether valsartan is distributed into human milk. Discontinue nursing or the drug because of potential risk in nursing infants.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2077
For more Drug Warnings (Complete) data for VALSARTAN (21 total), please visit the HSDB record page.
Valsartan is indicated for the treatment of hypertension to reduce the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. It is also indicated for the treatment of heart failure (NYHA class II-IV) and for left ventricular dysfunction or failure after myocardial infarction when the use of an angiotensin-converting enzyme inhibitor (ACEI) is not appropriate.
FDA Label
Treatment of essential hypertension.
Exforge is indicated in patients whose blood pressure is not adequately controlled on amlodipine or valsartan monotherapy.
Heart failure following recent myocardial infarction, Treatment of heart failure, Treatment of hypertension
Valsartan inhibits the pressor effects of angiotensin II with oral doses of 80 mg inhibiting the pressor effect by about 80% at peak with approximately 30% inhibition persisting for 24 hours. Removal of the negative feedback of angiotensin II causes a 2- to 3-fold rise in plasma renin and consequent rise in angiotensin II plasma concentration in hypertensive patients. Minimal decreases in plasma aldosterone were observed after administration of valsartan. In multiple-dose studies in hypertensive patients, valsartan had no notable effects on total cholesterol, fasting triglycerides, fasting serum glucose, or uric acid. **Hypotension** Excessive hypotension was rarely seen (0.1%) in patients with uncomplicated hypertension treated with valsartan alone. In patients with an activated renin-angiotensin system, such as volume- and/or salt-depleted patients receiving high doses of diuretics, symptomatic hypotension may occur. This condition should be corrected prior to administration of valsartan, or the treatment should start under close medical supervision. Caution should be observed when initiating therapy in patients with heart failure. Patients with heart failure given valsartan commonly have some reduction in blood pressure, but discontinuation of therapy because of continuing symptomatic hypotension usually is not necessary when dosing instructions are followed. In controlled trials in heart failure patients, the incidence of hypotension in valsartan-treated patients was 5.5% compared to 1.8% in placebo-treated patients. If excessive hypotension occurs, the patient should be placed in the supine position and, if necessary, given an intravenous infusion of normal saline. A transient hypotensive response is not a contraindication to further treatment, which usually can be continued without difficulty once the blood pressure has stabilized. **Impaired Renal Function** Changes in renal function including acute renal failure can be caused by drugs that inhibit the renin-angiotensin system and by diuretics. Patients whose renal function may depend in part on the activity of the renin-angiotensin system (e.g., patients with renal artery stenosis, chronic kidney disease, severe congestive heart failure, or volume depletion) may be at particular risk of developing acute renal failure on valsartan. Monitor renal function periodically in these patients. Consider withholding or discontinuing therapy in patients who develop a clinically significant decrease in renal function on valsartan. **Hyperkalemia** Some patients with heart failure have developed increases in potassium. These effects are usually minor and transient, and they are more likely to occur in patients with pre-existing renal impairment. Dosage reduction and/or discontinuation of valsartan may be required.
Angiotensin II Type 1 Receptor Blockers
Agents that antagonize ANGIOTENSIN II TYPE 1 RECEPTOR. Included are ANGIOTENSIN II analogs such as SARALASIN and biphenylimidazoles such as LOSARTAN. Some are used as ANTIHYPERTENSIVE AGENTS. (See all compounds classified as Angiotensin II Type 1 Receptor Blockers.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
C09DB01
C09CA03
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
C09CA03
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
C - Cardiovascular system
C09 - Agents acting on the renin-angiotensin system
C09C - Angiotensin ii receptor blockers (arbs), plain
C09CA - Angiotensin ii receptor blockers (arbs), plain
C09CA03 - Valsartan
Absorption
After one oral dose, the antihypertensive activity of valsartan begins within approximately 2 hours and peaks within 4-6 hours in most patients. Food decreases the exposure to orally administered valsartan by approximately 40% and peak plasma concentration by approximately 50%. AUC and Cmax values of valsartan genereally increase linearly with increasing dose over the therapeutic dose range. Valsartan does not accumulate appreciably in plasma following repetitive administration.
Route of Elimination
Valsartan, when administered as an oral solution, is primarily recovered in feces (about 83% of dose) and urine (about 13% of dose). The recovery is mainly as unchanged drug, with only about 20% of dose recovered as metabolites.
Volume of Distribution
The steady state volume of distribution of valsartan after intravenous administration is small (17 L), indicating that valsartan does not distribute into tissues extensively.
Clearance
Following intravenous administration, plasma clearance of valsartan is approximately 2 L/hour and its renal clearance is 0.62 L/hour (about 30% of total clearance).
Valsartan, when administered as an oral solution, is primarily recovered in feces (about 83% of dose) and urine (about 13% of dose). The recovery is mainly as unchanged drug, with only about 20% of dose recovered as metabolites. ... Following intravenous administration, plasma clearance of valsartan is about 2 L/hr and its renal clearance is 0.62 L/hr (about 30% of total clearance).
NIH; DailyMed. Current Medication Information for Diovan (Valsartan) Tablet (Revised: March 2014). Available from, as of May 28, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5ddba454-f3e6-43c2-a7a6-58365d297213
Absolute bioavailability for the capsule formulation is approximately 25% (range, 10-35%). Food decreases the area under the plasma concentration-time curve (AUC) and peak plasma concentration by approximately 40 and 50%, respectively.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2860
Valsartan peak plasma concentration is reached 2 to 4 hours after dosing. Valsartan shows bi-exponential decay kinetics following intravenous administration, with an average elimination half-life of about 6 hours. Absolute bioavailability for Diovan is about 25% (range 10% to 35%). The bioavailability of the suspension is 1.6 times greater than with the tablet. With the tablet, food decreases the exposure (as measured by AUC) to valsartan by about 40% and peak plasma concentration (Cmax) by about 50%. AUC and Cmax values of valsartan increase approximately linearly with increasing dose over the clinical dosing range. Valsartan does not accumulate appreciably in plasma following repeated administration.
NIH; DailyMed. Current Medication Information for Diovan (Valsartan) Tablet (Revised: March 2014). Available from, as of May 28, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5ddba454-f3e6-43c2-a7a6-58365d297213
The steady state volume of distribution of valsartan after intravenous administration is small (17 L), indicating that valsartan does not distribute into tissues extensively. Valsartan is highly bound to serum proteins (95%), mainly serum albumin.
NIH; DailyMed. Current Medication Information for Diovan (Valsartan) Tablet (Revised: March 2014). Available from, as of May 28, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5ddba454-f3e6-43c2-a7a6-58365d297213
For more Absorption, Distribution and Excretion (Complete) data for VALSARTAN (6 total), please visit the HSDB record page.
Valsartan undergoes minimal liver metabolism and is not biotransformed to a high degree, as only approximately 20% of a single dose is recovered as metabolites. The primary metabolite, accounting for about 9% of dose, is valeryl 4-hydroxy valsartan. In vitro metabolism studies involving recombinant CYP 450 enzymes indicated that the CYP 2C9 isoenzyme is responsible for the formation of valeryl-4-hydroxy valsartan. Valsartan does not inhibit CYP 450 isozymes at clinically relevant concentrations. CYP 450 mediated drug interaction between valsartan and coadministered drugs are unlikely because of the low extent of metabolism.
Valsartan is known to be excreted largely as unchanged compound and is minimally metabolized in man. Although the only notable metabolite is 4-hydroxyvaleryl metabolite (4-OH valsartan), the responsible enzyme has not been clarified at present. The current in vitro studies were conducted to identify the cytochrome P450 (CYP) enzymes involved in the formation of 4-OH valsartan. Valsartan was metabolized to 4-OH valsartan by human liver microsomes and the Eadie-Hofstee plots were linear. The apparent Km and Vmax values for the formation of 4-OH valsartan were 41.9-55.8 microM and 27.2-216.9 pmol min(-1) mg(-1) protein, respectively. There was good correlation between the formation rates of 4-OH valsartan and diclofenac 4'-hydroxylase activities (representative CYP2C9 activity) of 11 individual microsomes (r = 0.889). No good correlation was observed between any of the other CYP enzyme marker activities (CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4 and CYP4A). Among the recombinant CYP enzymes examined (CYPs 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A4, 3A5 and 4A11), CYP2C9 notably catalysed 4-hydroxylation of valsartan. For the specific CYP inhibitors or substrates examined (furafylline, diclofenac, S(+)-mephenytoin, quinidine and troleandomycin), only diclofenac inhibited the formation of 4-OH valsartan. These results showed that CYP2C9 is the only form responsible for 4-hydroxylation of valsartan in human liver microsomes. Although CYP2C9 is involved in valsartan metabolism, CYP-mediated drug-drug interaction between valsartan and other co-administered drugs would be negligible.
PMID:16192110 Nakashima A et al; Xenobiotica 35 (6): 589-602 (2005)
... Valsartan, when administered as an oral solution, is primarily recovered in feces (about 83% of dose) and urine (about 13% of dose). The recovery is mainly as unchanged drug, with only about 20% of dose recovered as metabolites. The primary metabolite, accounting for about 9% of dose, is valeryl 4-hydroxy valsartan. In vitro metabolism studies involving recombinant CYP 450 enzymes indicated that the CYP 2C9 isoenzyme is responsible for the formation of valeryl-4-hydroxy valsartan. Valsartan does not inhibit CYP 450 isozymes at clinically relevant concentrations. CYP 450 mediated drug interaction between valsartan and coadministered drugs are unlikely because of the low extent of metabolism. ...
NIH; DailyMed. Current Medication Information for Diovan (Valsartan) Tablet (Revised: March 2014). Available from, as of May 28, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5ddba454-f3e6-43c2-a7a6-58365d297213
Valsartan has known human metabolites that include 4-hydroxy-valsartan.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
After intravenous (IV) administration, valsartan demonstrates bi-exponential decay kinetics, with an average elimination half-life of about 6 hours.
Valsartan shows bi-exponential decay kinetics following intravenous administration, with an average elimination half-life of about 6 hours.
NIH; DailyMed. Current Medication Information for Diovan (Valsartan) Tablet (Revised: March 2014). Available from, as of May 28, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5ddba454-f3e6-43c2-a7a6-58365d297213
... In an investigation of pharmacokinetics and pharmacodynamics in normotensive male volunteers, valsartan was rapidly absorbed with the maximal plasma concentration occurring 2-3 hr after oral administration. The elimination half-life was about 4-6 hr, valsartan was poorly metabolized, and most of the drug was excreted via feces. ...
PMID:12491811 Kimura M et al; Nippon Yakurigaku Zasshi 120 (5): 353-60 (2002)
Valsartan belongs to the angiotensin II receptor blocker (ARB) family of drugs, which selectively bind to angiotensin receptor 1 (AT1) and prevent angiotensin II from binding and exerting its hypertensive effects. These include vasoconstriction, stimulation and synthesis of aldosterone and ADH, cardiac stimulation, and renal reabsorption of sodium among others. Overall, valsartan's physiologic effects lead to reduced blood pressure, lower aldosterone levels, reduced cardiac activity, and increased excretion of sodium. Valsartan also affects the renin-angiotensin aldosterone system (RAAS), which plays an important role in hemostasis and regulation of kidney, vascular, and cardiac functions. Pharmacological blockade of RAAS via AT1 receptor blockade inhibits negative regulatory feedback within RAAS which is a contributing factor to the pathogenesis and progression of cardiovascular disease, heart failure, and renal disease. In particular, heart failure is associated with chronic activation of RAAS, leading to inappropriate fluid retention, vasoconstriction, and ultimately a further decline in left ventricular function. ARBs have been shown to have a protective effect on the heart by improving cardiac function, reducing afterload, increasing cardiac output and prevent ventricular hypertrophy. The angiotensin-converting enzyme inhibitor (ACEI) class of medications (which includes drugs such as [ramipril], [lisinopril], and [perindopril]) inhibits the conversion of angiotensin I to angiotensin II by inhibiting the ACE enzyme but does not prevent the formation of all angiotensin II. ARB activity is unique in that it blocks all angiotensin II activity, regardless of where or how it was synthesized. Valsartan is commonly used for the management of hypertension, heart failure, and type 2 diabetes-associated nephropathy, particularly in patients who are unable to tolerate ACE inhibitors. ARBs such as valsartan have been shown in a number of large-scale clinical outcomes trials to improve cardiovascular outcomes including reducing risk of myocardial infarction, stroke, the progression of heart failure, and hospitalization. Valsartan also slows the progression of diabetic nephropathy due to its renoprotective effects. Improvements in chronic kidney disease with valsartan include both clinically and statistically significant decreases in urinary albumin and protein excretion in patients diagnosed with type 2 diabetes and in nondiabetic patients diagnosed with chronic kidney disease. Valsartan also binds to the AT2 receptor, however AT2 is not known to be associated with cardiovascular homeostasis like AT1. Valsartan has about 20,000-fold higher affinity for the AT1 receptor than for the AT2 receptor. The increased plasma levels of angiotensin II following AT1 receptor blockade with valsartan may stimulate the unblocked AT2 receptor.
Valsartan, a nonpeptide tetrazole derivative, is an angiotensin II type 1 (AT1) receptor antagonist. Valsartan has pharmacologic actions similar to those of losartan; however, unlike losartan, valsartan is not a prodrug and its pharmacologic activity does not depend on hydrolysis in the liver. Valsartan blocks the physiologic actions of angiotensin II, including vasoconstrictor and aldosterone-secreting effects, by selectively inhibiting access of angiotensin II to AT1 receptors within many tissues, including vascular smooth muscle and the adrenal gland. By comparison, angiotensin-converting enzyme (ACE, kininase II) inhibitors block the conversion of angiotensin I to angiotensin II; however, the blockade of angiotensin II production by ACE inhibitors is not complete since the vasopressor hormone can be formed via other enzymes that are not blocked by ACE inhibitors. Because valsartan, unlike ACE inhibitors, does not inhibit ACE, the drug does not interfere with response to bradykinins and substance P; a beneficial consequence is the absence of certain ACE inhibitor-induced adverse effects (e.g., cough), but possible renal and/or cardioprotective effects may be sacrificed. Valsartan also does not interfere with angiotensin II synthesis.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2077
Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system, with effects that include vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation, and renal reabsorption of sodium. Diovan (valsartan) blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in many tissues, such as vascular smooth muscle and the adrenal gland. Its action is therefore independent of the pathways for angiotensin II synthesis. There is also an AT2 receptor found in many tissues, but AT2 is not known to be associated with cardiovascular homeostasis. Valsartan has much greater affinity (about 20,000-fold) for the AT1 receptor than for the AT2 receptor. The increased plasma levels of angiotensin II following AT1 receptor blockade with valsartan may stimulate the unblocked AT2 receptor. The primary metabolite of valsartan is essentially inactive with an affinity for the AT1 receptor about one-200th that of valsartan itself. Blockade of the renin-angiotensin system with ACE inhibitors, which inhibit the biosynthesis of angiotensin II from angiotensin I, is widely used in the treatment of hypertension. ACE inhibitors also inhibit the degradation of bradykinin, a reaction also catalyzed by ACE. Because valsartan does not inhibit ACE (kininase II), it does not affect the response to bradykinin. Whether this difference has clinical relevance is not yet known. Valsartan does not bind to or block other hormone receptors or ion channels known to be important in cardiovascular regulation. Blockade of the angiotensin II receptor inhibits the negative regulatory feedback of angiotensin II on renin secretion, but the resulting increased plasma renin activity and angiotensin II circulating levels do not overcome the effect of valsartan on blood pressure.
NIH; DailyMed. Current Medication Information for Diovan (Valsartan) Tablet (Revised: March 2014). Available from, as of May 28, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=5ddba454-f3e6-43c2-a7a6-58365d297213
Recent studies demonstrated that the antihypertensive drug valsartan improved spatial and episodic memory in mouse models of Alzheimer's Disease (AD) and human subjects with hypertension. However, the molecular mechanism by which valsartan can regulate cognitive function is still unknown. The effect of valsartan on dendritic spine formation in primary hippocampal neurons, which is correlated with learning and memory /was investigated/. /It was/ found that valsartan promotes spinogenesis in developing and mature neurons. In addition, /it was found/ that valsartan increases the puncta number of PSD-95 and trends toward an increase in the puncta number of synaptophysin. Moreover, valsartan increased the cell surface levels of AMPA receptors and selectively altered the levels of spinogenesis-related proteins, including CaMKIIa and phospho-CDK5. These data suggest that valsartan may promote spinogenesis by enhancing AMPA receptor trafficking and synaptic plasticity signaling.
PMID:24012668 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3840727 Sohn YI et al; Biochem Biophys Res Commun 439 (4):464-70 (2013)
The mechanisms underlying the myocardial protection of valsartan against ischemia/reperfusion (I/R) injury are complicated and remain unclear. The aim of this study was to investigate whether autophagy machinery was involved in the protection against I/R injury that is induced by valsartan. In vivo rat hearts were subjected to ischemia by 30 min ligation of the left anterior descending coronary artery, followed by a 120 min reperfusion. 3-methyladenine (3-MA), a specific inhibitor on autophagic sequestration, was used to inhibit autophagy. The hemodynamics, infarct size of the ventricle and LC3B protein were measured. Western blot analysis was performed to investigate the mechanism by which autophagy was induced by valsartan. Valsartan preconditioning resulted in a significant decrease in infarct size and induced autophagy in the rat heart subjected to I/R injury. The hemodynamics assay showed that the valsartan-induced cardiac functional recovery was attenuated by 3-MA. By contrast, 3-MA decreased the improvement induced by valsartan on the histology and infarction of the rat heart subjected to I/R injury. Valsartan preconditioning induced autophagy via the AKT/mTOR/S6K pathway, independent of Beclin1. In conclusion, valsartan preconditioning induced autophagy via the AKT/mTOR/S6K pathway, which contributed to the myocardial protection against I/R injury.
PMID:24084854 Wu X et al; Mol Med Rep 8 (6): 1824-30 (2013)
For more Mechanism of Action (Complete) data for VALSARTAN (18 total), please visit the HSDB record page.








