

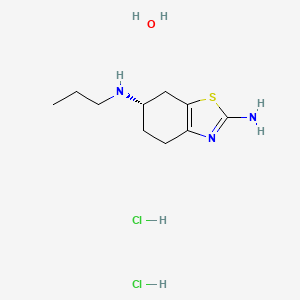

1. 2 Amino 6 Propylaminotetrahydrobenzothiazole
2. 2-amino-4,5,6,7-tetrahydro-6-propylaminobenzothiazole
3. 2-amino-6-propylaminotetrahydrobenzothiazole
4. 4,5,6,7-tetrahydro-n6-propyl-2,6-benzothiazole-diamine
5. 6,7-tetrahydro-n6-propyl-2,6-benzothiazolediamine Dihydrochloride Monohydrate
6. Dexpramipexole
7. Kns 760704
8. Kns-760704
9. Kns760704
10. Mirapex
11. Pramipexol
12. Pramipexol Dihydrobromide, (+-)-isomer
13. Pramipexol Dihydrochloride, (s)-isomer
14. Pramipexol, (+-)-isomer
15. Pramipexol, (r)-isomer
16. Pramipexole
17. Pramipexole Dihydrochloride
18. Pramipexole Dihydrochloride Anhydrous
19. Pramipexole Hydrochloride Monohydrate
20. Sifrol
21. Snd 919
22. Snd 919cl2x
23. Snd-919
24. Snd-919cl2x
25. Snd919cl2x
1. 191217-81-9
2. Mirapex
3. (s)-n6-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine Dihydrochloride Hydrate
4. Pramipexole 2hcl Monohydrate
5. Pramipexole Hydrochloride
6. Sifrol
7. Daquiran
8. Pramipexole Hydrochloride Hydrate
9. Pramipexole Teva
10. Pramipexole Accord
11. Snd919cl2y
12. Bi-sifrol
13. Pnu-98528e
14. 191712-81-9
15. (s)-2-amino-4,5,6,7-tetrahydro-6-(propylamino)benzothiazole Dihydrochloride Monohydrate
16. Pramipexole Dihydrochloride [usan]
17. Chebi:51147
18. 3d867np06j
19. Oprymea
20. Pramipexole (dihydrochloride Hydrate)
21. Snd-919cl2y
22. (6s)-6-n-propyl-4,5,6,7-tetrahydro-1,3-benzothiazole-2,6-diamine;hydrate;dihydrochloride
23. Pramipexole Hydrochloride Hydrate (jan)
24. Pramipexole Dihydrochloride Anhydrous
25. Pramipexole Hydrochloride Hydrate [jan]
26. Pramipexole Hcl Hydrate
27. Unii-3d867np06j
28. Pramipexole Dihydrochloride Hydrate
29. Pramipexole Hydrate Dihydrochloride
30. Pnu 98528e
31. Mirapex (tn)
32. Pramipexole Dihydrochloride [usan:usp]
33. Dsstox_cid_24227
34. Dsstox_rid_80129
35. Dsstox_gsid_44227
36. Chembl3182733
37. Dtxsid1044227
38. Hy-b0410a
39. Pramipexole Dihydrochloride (usp)
40. Pramipexole Hydrochloride Monohydrate
41. Tox21_302316
42. Mfcd02183927
43. S2011
44. Akos015917338
45. Ccg-267486
46. Ks-1308
47. 2,6-benzothiazolediamine, 4,5,6,7-tetrahydro-n6-propyl-, Dihydrochloride, Monohydrate, (6s)-
48. Ncgc00255978-01
49. Pramipexole Hydrochloride [mart.]
50. 112gi013
51. Bp162212
52. Bp164285
53. Pramipexole Dihydrochloride [vandf]
54. Cas-191217-81-9
55. Cs-0013154
56. Sw197453-5
57. (s)-pramipexole 2hcl Monohydrate - Ep Grade
58. Pramipexole Dihydrochloride Monohydrate- Bio-x
59. D00559
60. T72003
61. Pramipexole Dihydrochloride [orange Book]
62. A846638
63. Pramipexole Dihydrochloride [usp Monograph]
64. J-012354
65. Pramipexole Dihydrochloride Monohydrate [mi]
66. Q27888021
67. Pramipexole Dihydrochloride Monohydrate [ema Epar]
68. Pramipexole Dihydrochloride Monohydrate [usp-rs]
69. Pramipexole Dihydrochloride Monohydrate [who-dd]
70. Pramipexole Dihydrochloride Monohydrate [ep Monograph]
71. (6s)-n(6)-propyl-4,5,6,7-tetrahydro-1,3-benzothiazole-2,6-diamine Dihydrochloride Hydrate
72. (s)-2-amino-4,5,6,7-tetrahydro-6(propylamino)benzothiazole Dihydrochloride Monohydrate
73. (s)-n6-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine 2hcl Hydrate
74. (s)-n6-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diaminedihydrochloridehydrate
| Molecular Weight | 302.3 g/mol |
|---|---|
| Molecular Formula | C10H21Cl2N3OS |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | 301.0782389 g/mol |
| Monoisotopic Mass | 301.0782389 g/mol |
| Topological Polar Surface Area | 80.2 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 188 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 4 |
| 1 of 4 | |
|---|---|
| Drug Name | Mirapex |
| PubMed Health | Pramipexole (By mouth) |
| Drug Classes | Antiparkinsonian |
| Drug Label | MIRAPEX tablets contain pramipexole, a nonergot dopamine agonist. The chemical name of pramipexole dihydrochloride is (S)-2-amino-4,5,6,7-tetrahydro-6-(propylamino)benzothiazole dihydrochloride monohydrate. Its empirical formula is C10 H17 N3 S 2... |
| Active Ingredient | Pramipexole dihydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 0.5mg; 1mg; 0.25mg; 0.75mg; 1.5mg; 0.125mg |
| Market Status | Prescription |
| Company | Boehringer Ingelheim |
| 2 of 4 | |
|---|---|
| Drug Name | Pramipexole dihydrochloride |
| Drug Label | MIRAPEX tablets contain pramipexole, a nonergot dopamine agonist. The chemical name of pramipexole dihydrochloride is (S)-2-amino-4,5,6,7-tetrahydro-6-(propylamino)benzothiazole dihydrochloride monohydrate. Its empirical formula is C10 H17 N3 S 2... |
| Active Ingredient | Pramipexole dihydrochloride |
| Dosage Form | Tablet, extended release; Tablet |
| Route | Oral |
| Strength | 0.5mg; 1mg; 0.25mg; 0.75mg; 3.75mg; 1.5mg; 4.5mg; 2.25mg; 0.375mg; 3mg; 0.125mg |
| Market Status | Prescription |
| Company | Anchen Pharms; Alembic; Breckenridge Pharm; Apotex; Aurobindo Pharma; Sun Pharm Inds; Torrent Pharms; Sandoz; Actavis Grp Ptc; Watson Labs; Strides Pharma; Glenmark Generics; Teva Pharms; Macleods Pharms; Zydus Pharms Usa; Mylan; Barr |
| 3 of 4 | |
|---|---|
| Drug Name | Mirapex |
| PubMed Health | Pramipexole (By mouth) |
| Drug Classes | Antiparkinsonian |
| Drug Label | MIRAPEX tablets contain pramipexole, a nonergot dopamine agonist. The chemical name of pramipexole dihydrochloride is (S)-2-amino-4,5,6,7-tetrahydro-6-(propylamino)benzothiazole dihydrochloride monohydrate. Its empirical formula is C10 H17 N3 S 2... |
| Active Ingredient | Pramipexole dihydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 0.5mg; 1mg; 0.25mg; 0.75mg; 1.5mg; 0.125mg |
| Market Status | Prescription |
| Company | Boehringer Ingelheim |
| 4 of 4 | |
|---|---|
| Drug Name | Pramipexole dihydrochloride |
| Drug Label | MIRAPEX tablets contain pramipexole, a nonergot dopamine agonist. The chemical name of pramipexole dihydrochloride is (S)-2-amino-4,5,6,7-tetrahydro-6-(propylamino)benzothiazole dihydrochloride monohydrate. Its empirical formula is C10 H17 N3 S 2... |
| Active Ingredient | Pramipexole dihydrochloride |
| Dosage Form | Tablet, extended release; Tablet |
| Route | Oral |
| Strength | 0.5mg; 1mg; 0.25mg; 0.75mg; 3.75mg; 1.5mg; 4.5mg; 2.25mg; 0.375mg; 3mg; 0.125mg |
| Market Status | Prescription |
| Company | Anchen Pharms; Alembic; Breckenridge Pharm; Apotex; Aurobindo Pharma; Sun Pharm Inds; Torrent Pharms; Sandoz; Actavis Grp Ptc; Watson Labs; Strides Pharma; Glenmark Generics; Teva Pharms; Macleods Pharms; Zydus Pharms Usa; Mylan; Barr |
Sifrol is indicated for treatment of the signs and symptoms of idiopathic Parkinson's disease, alone (without levodopa) or in combination with levodopa, i. e. over the course of the disease, though to late stages when the effect of levodopa wears off or becomes inconsistent and fluctuations of the therapeutic effect occur (end-of-dose or 'on-off' fluctuations).
Sifrol is indicated for symptomatic treatment of moderate to severe idiopathic restless-legs syndrome in dosages up to 0. 54 mg of base (0. 75 mg of salt).
Mirapexin is indicated for treatment of the signs and symptoms of idiopathic Parkinson's disease, alone (without levodopa) or in combination with levodopa, i. e. over the course of the disease, through to late stages when the effect of levodopa wears off or becomes inconsistent and fluctuations of the therapeutic effect occur (end-of-dose or 'on-off' fluctuations).
Mirapexin is indicated for symptomatic treatment of moderate to severe idiopathic restless-legs syndrome in dosages up to 0. 54 mg of base (0. 75 mg of salt).
Oprymea is indicated for treatment of the signs and symptoms of idiopathic Parkinson's disease, alone (without levodopa) or in combination with levodopa, i. e. over the course of the disease, through to late stages when the effect of levodopa wears off or becomes inconsistent and fluctuations of the therapeutic effect occur (end of dose or "on off" fluctuations).
Oprymea is indicated in adults for symptomatic treatment of moderate to severe idiopathic Restless Legs Syndrome in doses up to 0. 54 mg of base (0. 75 mg of salt) (see section 4. 2).
Pramipexole Teva is indicated for treatment of the signs and symptoms of idiopathic Parkinson's disease, alone (without levodopa) or in combination with levodopa, i. e. over the course of the disease, through to late stages when the effect of levodopa wears off or becomes inconsistent and fluctuations of the therapeutic effect occur (end-of-dose or 'on-off' fluctuations).
Pramipexole Teva is indicated in adults for symptomatic treatment of moderate to severe idiopathic Restless Legs Syndrome in doses up to 0. 54 mg of base (0. 75 mg of salt) (see section 4. 2).
Pramipexole Accord is indicated in adults for treatment of the signs and symptoms of idiopathic Parkinson's disease, alone (without levodopa) or in combination with levodopa, i. e. over the course of the disease, through to late stages when the effect of levodopa wears off or becomes inconsistent and fluctuations of the therapeutic effect occur (end-of-dose or 'on-off' fluctuations).
DAQUIRAN tablets are indicated for treatment of the signs and symptoms of advanced idiopathic Parkinson's disease in combination with levodopa, i. e. over the course of the disease, when the effect of levodopa wears off or becomes inconsistent and fluctuations of the therapeutic effect occur (end of dose or "on off" fluctuations).
Combined vocal and multiple motor tic disorder (de la Tourette), Restless Legs Syndrome
Combined vocal and multiple motor tic disorder (de la Tourette), Restless Legs Syndrome
Antiparkinson Agents
Agents used in the treatment of Parkinson's disease. The most commonly used drugs act on the dopaminergic system in the striatum and basal ganglia or are centrally acting muscarinic antagonists. (See all compounds classified as Antiparkinson Agents.)
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)
Dopamine Agonists
Drugs that bind to and activate dopamine receptors. (See all compounds classified as Dopamine Agonists.)
N04BC05
N04BC05
N04BC05
N04BC05
N04BC05
N04BC05