
1. Akamin
2. Akne Puren
3. Akne-puren
4. Aknemin
5. Aknin Mino
6. Aknin-mino
7. Aknosan
8. Apo Minocycline
9. Apo-minocycline
10. Arestin
11. Blemix
12. Cyclomin
13. Cyclops
14. Dentomycin
15. Dynacin
16. Hydrochloride, Minocycline
17. Icht Oral
18. Icht-oral
19. Klinomycin
20. Lederderm
21. Mestacine
22. Minakne
23. Mino Wolff
24. Mino-wolff
25. Minocin
26. Minocin Mr
27. Minoclir
28. Minocycline
29. Minocycline Monohydrochloride
30. Minocycline, (4r-(4 Alpha,4a Beta,5a Beta,12a Beta))-isomer
31. Minolis
32. Minomycin
33. Minoplus
34. Minotab
35. Minox 50
36. Monohydrochloride, Minocycline
37. Mynocine
1. 13614-98-7
2. Minocycline Hcl
3. Arestin
4. Solodyn
5. Minomycin
6. Minocin
7. Dynacin
8. Periocline
9. Ximino
10. Minocycline (hydrochloride)
11. Vectrin
12. Minomax
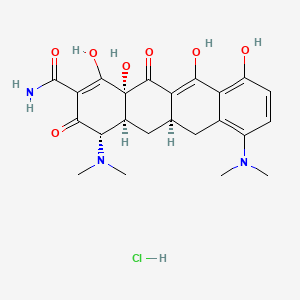
13. (4s,4as,5ar,12as)-4,7-bis(dimethylamino)-3,10,12,12a-tetrahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide Hydrochloride
14. Mynocine Hydrochloride
15. Minocycline (as Hydrochloride)
16. Minocycline, Hydrochloride
17. 4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide Monohydrochloride
18. 0020414e5u
19. Tri-mino
20. Nsc-141993
21. Dsstox_cid_24545
22. Dsstox_rid_80306
23. Dsstox_gsid_44545
24. (4s,4as,5ar,12ar)-4,7-bis(dimethylamino)-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide;hydrochloride
25. [4s-(4alpha,4aalpha,5aalpha,12aalpha)]-4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxonaphthacene-2-carboxamide Monohydrochloride
26. Lederderm
27. Mynocine
28. Acnez
29. Minomycin Chloride
30. Chebi:50697
31. (4s-(4alpha,4aalpha,5aalpha,12aalpha))-4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxonaphthacene-2-carboxamide Monohydrochloride
32. [4s-(4?,4a?,5a?,12a?)]-4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a,tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide
33. 2-naphthacenecarboxamide, 4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-, Hydrochloride (1:2), (4s,4as,5ar,12as)-
34. Einecs 237-099-7
35. Minocycline Hydrochloride (internal Use)
36. C23h27n3o7.hcl
37. Minocycline, Hcl
38. Unii-0020414e5u
39. Ncgc00096006-01
40. Prestwick_626
41. Arestin (tn)
42. Dynacin (tn)
43. Minocin (tn)
44. Solodyn (tn)
45. Minocycline Hydrochloride [usp:jan]
46. 7-dimethylamino-6-demethyl-6-deoxytetracycline, Hcl
47. Mfcd00083669
48. Minocyclinhydrochlorid
49. Amzeeq
50. Minolira
51. Zilxi
52. Minomycin Hydrochloride
53. Schembl2537
54. Cas-13614-98-7
55. 2-naphthacenecarboxamide, 4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-, Monohydrochloride, (4s-(4alpha,4aalpha,5aalpha,12aalpha))-
56. Mls002548863
57. Spectrum1500414
58. Fxfm-244
59. Chembl1200881
60. Chembl4576719
61. Dtxsid8044545
62. Hms1568p12
63. Pharmakon1600-01500414
64. Bcp06597
65. Tox21_111250
66. Tox21_113183
67. Tox21_301590
68. Ccg-40107
69. Nsc757120
70. S4226
71. Minocycline Hydrochloride [mi]
72. Minocycline Hydrochloride, Crystalline
73. Akos015951312
74. Minocycline Hydrochloride (jp17/usp)
75. Tox21_111250_1
76. Cs-1256
77. Minocycline Hydrochloride [jan]
78. Nc00460
79. Nsc-757120
80. Minocycline Hydrochloride [mart.]
81. Minocycline Hydrochloride [vandf]
82. Ncgc00178854-03
83. Ncgc00255988-01
84. Ac-22362
85. As-75306
86. Hy-17412
87. Minocycline Hydrochloride [usp-rs]
88. Minocycline Hydrochloride [who-dd]
89. Smr001906766
90. M2288
91. D00850
92. Minocycline Hydrochloride [orange Book]
93. Minocycline Hydrochloride [usp Monograph]
94. 614m987
95. Sr-01000075625
96. Q-201407
97. Sr-01000075625-1
98. Q27104777
99. Minocycline Hydrochloride, Antibiotic For Culture Media Use Only
100. Minocycline Hydrochloride, European Pharmacopoeia (ep) Reference Standard
101. Minocycline Hydrochloride, Pharmaceutical Secondary Standard; Certified Reference Material
102. Minocycline Hydrochloride, United States Pharmacopeia (usp) Reference Standard
103. (4s,12as,4as,5ar)-4,7-bis(dimethylamino)-3,10,12,12a-tetrahydroxy-1,11-dioxo-4,5,6,12a,4a,5a-hexahydronaphthacene-2-carboxamide Hydrochloride
104. (4s,4as,5ar,12as)-4,7-bis(dimethylamino)-3,10,12,12a-tetrahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamidehydrochloride
105. 2-naphthacenecarboxamide, 4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-, Monohydrochloride, (4s-(4.alpha.,4a.alpha.,5a.alpha.,12a.alpha.))-
| Molecular Weight | 493.9 g/mol |
|---|---|
| Molecular Formula | C23H28ClN3O7 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 3 |
| Exact Mass | 493.1615779 g/mol |
| Monoisotopic Mass | 493.1615779 g/mol |
| Topological Polar Surface Area | 165 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 971 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 10 | |
|---|---|
| Drug Name | Arestin |
| PubMed Health | Minocycline |
| Drug Classes | Amebicide, Intestinal, Antiacne, Antiacne Antibacterial, Antibiotic, Antiprotozoal, Tetracycline |
| Drug Label | ARESTIN (minocycline hydrochloride) Microspheres is a subgingival sustained-release product containing the antibiotic minocycline hydrochloride incorporated into a bioresorbable polymer, Poly (glycolide-co-dl-lactide) or PGLA, for professional subg... |
| Active Ingredient | Minocycline hydrochloride |
| Dosage Form | Powder, extended release |
| Route | Dental |
| Strength | eq 1mg base |
| Market Status | Prescription |
| Company | Orapharma |
| 2 of 10 | |
|---|---|
| Drug Name | Dynacin |
| Drug Label | Minocycline hydrochloride, is a semisynthetic derivative of tetracycline, 4,7-Bis(dimethylamino)1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1, 11-dioxo-2naphthacenecarboxamide monohydrochloride. Its structural formula is:C23H27N3O7... |
| Active Ingredient | Minocycline hydrochloride |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 100mg base; eq 75mg base |
| Market Status | Prescription |
| Company | Cnty Line Pharms |
| 3 of 10 | |
|---|---|
| Drug Name | Minocin |
| PubMed Health | Minocycline, Regular Release (By mouth) |
| Drug Classes | Amebicide, Intestinal, Antiacne, Antibiotic, Antiprotozoal |
| Drug Label | MINOCIN minocycline hydrochloride, is a semisynthetic derivative of tetracycline, 4,7-Bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide monohydrochloride.Its structural formula is:C23H27N... |
| Active Ingredient | Minocycline hydrochloride |
| Dosage Form | Capsule; Injectable |
| Route | Injection; Oral |
| Strength | eq 100mg base; eq 50mg base; eq 100mg base/vial |
| Market Status | Prescription |
| Company | Rempex Pharms; Precision Dermat |
| 4 of 10 | |
|---|---|
| Drug Name | Minocycline hydrochloride |
| Drug Label | To reduce the development of drug-resistant bacteria and maintain the effectiveness of minocycline hydrochloride capsules and other antibacterial drugs, minocycline hydrochloride capsules should be used only to treat or prevent infections that are pr... |
| Active Ingredient | Minocycline hydrochloride |
| Dosage Form | Tablet, extended release; Tablet; Capsule |
| Route | oral; Oral |
| Strength | 65mg; 115mg; eq 100mg base; eq 50mg base; eq 105mg base; eq 65mg base; eq 115mg base; eq 75mg base; eq 90mg base; eq 80mg base; 55mg; eq 45mg base; eq 55mg base; eq 135mg base |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharms; Ranbaxy; Teva; Mylan Labs; Sun Pharm Inds; Aurobindo Pharma; Lupin; Barr Labs; Sandoz; Par Pharm; Watson Labs; Ranbaxy Labs; Dr Reddys Labs; Impax Labs |
| 5 of 10 | |
|---|---|
| Drug Name | Solodyn |
| PubMed Health | Minocycline |
| Drug Classes | Amebicide, Intestinal, Antiacne, Antiacne Antibacterial, Antibiotic, Antiprotozoal, Tetracycline |
| Drug Label | Minocycline hydrochloride, a semi synthetic derivative of tetracycline, is [4S-(4,4a,5a,12a)]-4,7-Bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide mono hydrochloride. The structura... |
| Active Ingredient | Minocycline hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | eq 105mg base; eq 65mg base; eq 115mg base; eq 80mg base; eq 55mg base |
| Market Status | Prescription |
| Company | Medicis |
| 6 of 10 | |
|---|---|
| Drug Name | Arestin |
| PubMed Health | Minocycline |
| Drug Classes | Amebicide, Intestinal, Antiacne, Antiacne Antibacterial, Antibiotic, Antiprotozoal, Tetracycline |
| Drug Label | ARESTIN (minocycline hydrochloride) Microspheres is a subgingival sustained-release product containing the antibiotic minocycline hydrochloride incorporated into a bioresorbable polymer, Poly (glycolide-co-dl-lactide) or PGLA, for professional subg... |
| Active Ingredient | Minocycline hydrochloride |
| Dosage Form | Powder, extended release |
| Route | Dental |
| Strength | eq 1mg base |
| Market Status | Prescription |
| Company | Orapharma |
| 7 of 10 | |
|---|---|
| Drug Name | Dynacin |
| Drug Label | Minocycline hydrochloride, is a semisynthetic derivative of tetracycline, 4,7-Bis(dimethylamino)1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1, 11-dioxo-2naphthacenecarboxamide monohydrochloride. Its structural formula is:C23H27N3O7... |
| Active Ingredient | Minocycline hydrochloride |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 100mg base; eq 75mg base |
| Market Status | Prescription |
| Company | Cnty Line Pharms |
| 8 of 10 | |
|---|---|
| Drug Name | Minocin |
| PubMed Health | Minocycline, Regular Release (By mouth) |
| Drug Classes | Amebicide, Intestinal, Antiacne, Antibiotic, Antiprotozoal |
| Drug Label | MINOCIN minocycline hydrochloride, is a semisynthetic derivative of tetracycline, 4,7-Bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide monohydrochloride.Its structural formula is:C23H27N... |
| Active Ingredient | Minocycline hydrochloride |
| Dosage Form | Capsule; Injectable |
| Route | Injection; Oral |
| Strength | eq 100mg base; eq 50mg base; eq 100mg base/vial |
| Market Status | Prescription |
| Company | Rempex Pharms; Precision Dermat |
| 9 of 10 | |
|---|---|
| Drug Name | Minocycline hydrochloride |
| Drug Label | To reduce the development of drug-resistant bacteria and maintain the effectiveness of minocycline hydrochloride capsules and other antibacterial drugs, minocycline hydrochloride capsules should be used only to treat or prevent infections that are pr... |
| Active Ingredient | Minocycline hydrochloride |
| Dosage Form | Tablet, extended release; Tablet; Capsule |
| Route | oral; Oral |
| Strength | 65mg; 115mg; eq 100mg base; eq 50mg base; eq 105mg base; eq 65mg base; eq 115mg base; eq 75mg base; eq 90mg base; eq 80mg base; 55mg; eq 45mg base; eq 55mg base; eq 135mg base |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharms; Ranbaxy; Teva; Mylan Labs; Sun Pharm Inds; Aurobindo Pharma; Lupin; Barr Labs; Sandoz; Par Pharm; Watson Labs; Ranbaxy Labs; Dr Reddys Labs; Impax Labs |
| 10 of 10 | |
|---|---|
| Drug Name | Solodyn |
| PubMed Health | Minocycline |
| Drug Classes | Amebicide, Intestinal, Antiacne, Antiacne Antibacterial, Antibiotic, Antiprotozoal, Tetracycline |
| Drug Label | Minocycline hydrochloride, a semi synthetic derivative of tetracycline, is [4S-(4,4a,5a,12a)]-4,7-Bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide mono hydrochloride. The structura... |
| Active Ingredient | Minocycline hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | eq 105mg base; eq 65mg base; eq 115mg base; eq 80mg base; eq 55mg base |
| Market Status | Prescription |
| Company | Medicis |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)