
Synopsis
Synopsis
0
CEP/COS
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
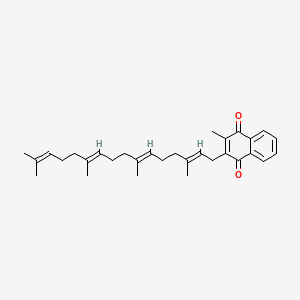

1. (e,e,e)-isomer Of Menatetrenone
2. 2-methyl-3-all-trans-tetraprenyl-1,4-naphthoquinone
3. 2-methyl-3-trans-tetraprenyl-1,4-naphthoquinone
4. Kefton-2
5. Menaquinone 4
6. Menaquinone-4
7. Vitamin K2(20)
8. Vitamin Mk-4
1. Menaquinone-4
2. Vitamin K2
3. 863-61-6
4. Menaquinone 4
5. 11032-49-8
6. Kaytwo
7. Kefton-2
8. Menaquinone K4
9. Vitamin Mk 4
10. Vitamin K2(20)
11. Menatetrenona
12. Menatetrenonum
13. Menatetranone
14. Mk-4
15. Kaytwo N
16. 2-methyl-3-[(2e,6e,10e)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraenyl]naphthalene-1,4-dione
17. 6041-00-5
18. 2-methyl-3-trans-tetraprenyl-1,4-naphthoquinone
19. 2-methyl-3-geranylgeranyl-1,4-naphthoquinone
20. Mls000028742
21. 2-methyl-3-((2e,6e,10e)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraen-1-yl)naphthalene-1,4-dione
22. Chebi:78277
23. 2-methyl-3-(3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl)-1,4-naphthoquinone
24. Smr000058955
25. 27y876d139
26. 1,4-naphthalenedione, 2-methyl-3-(3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl)-
27. Dsstox_cid_28895
28. 1,4-naphthalenedione, 2-methyl-3-((2e,6e,10e)3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl)-
29. Vitamin K2(sub 20)
30. Menatetrenone [inn:jan]
31. Mk4
32. Menatetrenonum [inn-latin]
33. K2(sub 20)
34. Menatetrenona [inn-spanish]
35. Glakay
36. E3100
37. Menaquinone(4)
38. Unii-27y876d139
39. Ncgc00183125-01
40. 2-methyl-3-((2e,6e,10e)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraenyl)naphthalene-1,4-dione
41. Mfcd00079646
42. Vitamin K2 (tn)
43. Opera_id_148
44. 2-methyl-3-tetraprenyl-1,4-naphthoquinone
45. Menatetrenone [inn]
46. Menatetrenone [jan]
47. 2-methyl-3-(3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl)-1,4-naphthochinon
48. Dsstox_rid_80857
49. Dsstox_rid_83163
50. Menatetrenone (jp17/inn)
51. Dsstox_gsid_45406
52. Dsstox_gsid_48969
53. Menaquinone 4 [mi]
54. 1,4-naphthalenedione, 2-methyl-3-(3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl)-, (e,e,e)-
55. Menaquinone;vitamin K2
56. Menatetrenone [mart.]
57. Schembl434553
58. Schembl571912
59. Menatetrenone [who-dd]
60. Vitamin K2 (mk-4) Solution
61. Chembl259223
62. Mq-4
63. Dtxsid6048969
64. Menaquinone-4 [usp-rs]
65. Hms2230j06
66. Ea-0167
67. Amy22672
68. Hy-b2156
69. Zinc3874199
70. Tox21_110567
71. Tox21_113413
72. Bdbm50423776
73. S5082
74. Akos025311021
75. Akos037645019
76. Menaquinone (k2), Analytical Standard
77. Ccg-269176
78. Db12148
79. 2-methyl-3-(3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl)-1,4-naphthalenedione
80. 2-methyl-3-(3,7,11,15-tetramethyl-2,6,10,14-hexadecatetrenyl)-1,4-naphthoquinone
81. Ncgc00181325-01
82. Ncgc00181325-03
83. As-17910
84. As-56161
85. Cas-863-61-6
86. Cs-0020307
87. M2398
88. A17083
89. D00100
90. E-0167
91. 863v616
92. A935204
93. Q192354
94. 1,4-naphthalenedione, 2-methyl-3-[(2e,6e,10e)-3,7,11,15-tetramethyl-2,6,10,14-hexadecatetraenyl]-
95. 2-methyl-3-[(2e,6e,10e)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraen-1-yl]-1,4-naphthoquinone
96. Vitamin K2 (mk-4) Solution, 100 Mug/ml In Acetonitrile, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 444.6 g/mol |
|---|---|
| Molecular Formula | C31H40O2 |
| XLogP3 | 8.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 11 |
| Exact Mass | 444.302830514 g/mol |
| Monoisotopic Mass | 444.302830514 g/mol |
| Topological Polar Surface Area | 34.1 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 855 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 3 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
There is no typical dosage for vitamin K. Some multivitamin preparations contain vitamin K as vitamin K1 (phylloquinone or phytonadione) or vitamin K2 (menaquinones) at doses of 25 to 100 ug.
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 711
Vitamin K is used to treat anticoagulant-induced prothrombin deficiency caused by warfarin, hyporprothrombinemia secondary to antibiotic therapy and hypoprothrombinemia secondary to vitamin C deficiency from various causes, including malabsorption syndromes. /Vitamin K/
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 709
Because hemorrhagic disease of the newborn can be effectively prevented by administrating vitamin K, infants born in the US and Canada routinely receive 0.5-1 mg pf phylloquinone intramuscularly or 2.0 mg orally within 6 hours of birth. This practice is supported by both US and Canadian pediatric societies. /Phylloquinone/
National Academies of Sciences Institute of Medicine; Vitamin K. In: Dietary Reference Intakes. p.255-61 (2006) National Academies Press, Washington, DC
The current recommendations of the American Academy of Pediatrics advise that "vitamin K (phylloquinone) should be given to all newborns as a single, intramuscular dose of 0.5-1 mg" and if this advice is followed, the disease /Vitamin K deficiency bleeding/ is effectively prevented. /Vitamin K/
Suttie JW; Vitamin K. In: Encyclopedia of Dietary Supplements, ed. Coates PM et al; Marcel Dekker, New York, NY pp. 771-82 (2005)
For more Therapeutic Uses (Complete) data for Vitamin K2 (8 total), please visit the HSDB record page.
... MK-7 induced more complete carboxylation of osteocalcin, and hematologists should be aware that preparations supplying 50 ug/d or more of MK-7 may interfere with oral anticoagulant treatment in a clinically relevant way.
PMID:17158229 Schurgers LJ et al; Blood 109 (8): 3279-83 (2007)
It has been suggested that vitamin K may have roles in osteoporosis and vascular health. However, this is difficult to establish on the basis of the studies performed thus far. /Vitamin K/
National Academies of Sciences Institute of Medicine; Vitamin K. In: Dietary Reference Intakes. p.255-61 (2006) National Academies Press, Washington, DC
Pregnant women and nursing mothers should avoid supplemental intakes of vitamin K greater than RDA amounts (65 ug daily) unless higher amounts are prescribed by their physicians. /Vitamin K/
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 711
Individuals on chronic warfarin therapy may require dietary counseling on how to maintain steady vitamin K intake levels. Because habitual vitamin K intake may modulate warfarin dosage in patients using this anticoagulant, these individuals should maintain their normal dietary and supplementation patterns once an effective dose of warfarin has been established. /Vitamin K/
National Academies of Sciences Institute of Medicine; Vitamin K. In: Dietary Reference Intakes. p.255-61 (2006) National Academies Press, Washington, DC
For more Drug Warnings (Complete) data for Vitamin K2 (6 total), please visit the HSDB record page.
Hemostatics
Agents acting to arrest the flow of blood. Absorbable hemostatics arrest bleeding either by the formation of an artificial clot or by providing a mechanical matrix that facilitates clotting when applied directly to the bleeding surface. These agents function more at the capillary level and are not effective at stemming arterial or venous bleeding under any significant intravascular pressure. (See all compounds classified as Hemostatics.)
Vitamin K, mainly in the form of vitamin K1, is principally absorbed from the jejunum and ileum. ... Vitamin K is delivered to the enterocytes in micelles formed from bile salts and other substances. Vitamin K is secreted by enterocytes into the lymphatics in the form of chylomicrons. It enters the circulation via the thoracic duct and is carried in the circulation to various tissues including hepatic, bone and spleen, in the form of chylomicron remnants. In the liver, some vitamin K is stored, some is oxidized to inactive end products and some is secreted with VLDL (very low density lipoprotein). Approximately 50% of vitamin K is carried in the plasma in the form of VLDL, about 25% in LDL (low-density lopoprotein) and about 25% in HDL (high-density lipoprotein). /Vitamin K/
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 709
Excretion of vitamin K and its metabolites is mainly via the feces. Some urinary excretion of vitamin K also occurs. /Vitamin K/
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 710
...Menaquinones are adequately absorbed from the GI tract only if bile salts are present. Menaquinones and its water-soluble derivatives, however, are absorbed even in the absence of bile. ... Menaquinones are absorbed almost entirely by way of the lymph. /Menaquinones/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1565
Menaquinone forms of vitamin K are produced by bacteria in the lower bowel, where the forms appear in large amounts. However, their contributuion to the maintenance of vitamin K status has been difficult to assess. Although the content is extremely variable, the human liver contains about 10 times as much vitamin K as a mixture of menaquinones than as phylloquinone. /Menaquinones/
National Academies of Sciences Institute of Medicine; Vitamin K. In: Dietary Reference Intakes. p.255-61 (2006) National Academies Press, Washington, DC
A major pathway of vitamin K metabolism is that which is involved in the reduction and recycling of the epoxide formed by the carboxylase. /Vitamin K/
Suttie JW; Vitamin K. In: Encyclopedia of Dietary Supplements, ed. Coates PM et al; Marcel Dekker, New York, NY pp. 771-82 (2005)
Vitamin K undergoes some oxidative metabolism. /Vitamin K/
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 710
In vivo and in vitro studies have shown that vitamin K may directly act on bone metabolism. In vitro studies have demonstrated that vitamin K2 inhibits bone resorption by, in part, inhibiting the production of bone resorbing substances such as prostaglandin E2 and interleukin-6. Vitamin K2 has been reported to enhance human osteoblast-induced mineralization in vitro and to inhibit bone loss in steroid-treated rats and ovariectomized rats.
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 709
Certain naphthoquinones, in particular the synthetic vitamin K menadione, have been found to have antitumor activity in vitro and in vivo. Vitamin K2 has been found to induce the in vitro differentiation of myeloid leukemic cell lines. The mechanism of the possible anticarcinogenic activity of vitamin K is not well understood. Menadione is an oxidative stress inducer and its possible anticarcinogenic activity may, in part, be explained by induction of apoptotic cell death. One study suggested that the induction of apoptosis by menadione is mediated by the Fas/Fas ligand system. Another study reported that menadione induces cell cycle arrest and cell death by inhibiting Cda 25 phosphatase.
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 709
Vitamin K is involved as a cofactor in the posttranslational gamma-carboxylation of glutamic acid residues of certain proteins in the body. These proteins include the vitamin K-dependent coagulation factors II (prothrombin), VII (proconvertin), IX (Christmas factor), X (Stuart factor), protein C, protein S, protein Zv and a growth-arrest-specific factor (Gas6). In contrast to the other vitamin K-dependent proteins in the blood coagulation cascade, protein C and protein X serve anticoagulant roles. The two vitamin K-dependent proteins found in bone are osteocalcin, also known as bone G1a (gamma-carboxyglutamate) protein or BGP, and the matrix G1a protein or MGP. Gamma-carboxylation is catalyzed by the vitamin K-dependent gamma-carboxylases. /Vitamin K/
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 708
The primary gene product of the vitamin K-dependent proteins contains a very homologous domain between the amino terminus of the mature protein and the signal sequence that targets the polypeptide for the secretory pathway. This "propeptide" region appears to be both a "docking" or "recognition " site for the enzyme and a modulator of the activity of the enzyme by decreasing the apparent Km of the Glu site substrate. ... A key finding essential to a complete understanding of the detailed mechanism of action of this enzyme has been the identification of an intermediate chemical form of vitamin K, which could be sufficiently basic to abstract the gamma-hydrogen of the glutamyl residue. It has been proposed that the initial attack of O(2) at the naphthoquinone carbonyl carbon adjacent to the methyl group results in the formation of a dioxetane ring, which generates an alkoxide intermediate. /Vitamin K/
Suttie JW; Vitamin K. In: Encyclopedia of Dietary Supplements, ed. Coates PM et al; Marcel Dekker, New York, NY pp. 771-82 (2005)
For more Mechanism of Action (Complete) data for Vitamin K2 (8 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
73
PharmaCompass offers a list of Vitamin K2 API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Vitamin K2 manufacturer or Vitamin K2 supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Vitamin K2 manufacturer or Vitamin K2 supplier.
PharmaCompass also assists you with knowing the Vitamin K2 API Price utilized in the formulation of products. Vitamin K2 API Price is not always fixed or binding as the Vitamin K2 Price is obtained through a variety of data sources. The Vitamin K2 Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Menatetrenone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Menatetrenone, including repackagers and relabelers. The FDA regulates Menatetrenone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Menatetrenone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Menatetrenone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Menatetrenone supplier is an individual or a company that provides Menatetrenone active pharmaceutical ingredient (API) or Menatetrenone finished formulations upon request. The Menatetrenone suppliers may include Menatetrenone API manufacturers, exporters, distributors and traders.
click here to find a list of Menatetrenone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Menatetrenone DMF (Drug Master File) is a document detailing the whole manufacturing process of Menatetrenone active pharmaceutical ingredient (API) in detail. Different forms of Menatetrenone DMFs exist exist since differing nations have different regulations, such as Menatetrenone USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Menatetrenone DMF submitted to regulatory agencies in the US is known as a USDMF. Menatetrenone USDMF includes data on Menatetrenone's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Menatetrenone USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Menatetrenone suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Menatetrenone Drug Master File in Japan (Menatetrenone JDMF) empowers Menatetrenone API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Menatetrenone JDMF during the approval evaluation for pharmaceutical products. At the time of Menatetrenone JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Menatetrenone suppliers with JDMF on PharmaCompass.
A Menatetrenone written confirmation (Menatetrenone WC) is an official document issued by a regulatory agency to a Menatetrenone manufacturer, verifying that the manufacturing facility of a Menatetrenone active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Menatetrenone APIs or Menatetrenone finished pharmaceutical products to another nation, regulatory agencies frequently require a Menatetrenone WC (written confirmation) as part of the regulatory process.
click here to find a list of Menatetrenone suppliers with Written Confirmation (WC) on PharmaCompass.
Menatetrenone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Menatetrenone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Menatetrenone GMP manufacturer or Menatetrenone GMP API supplier for your needs.
A Menatetrenone CoA (Certificate of Analysis) is a formal document that attests to Menatetrenone's compliance with Menatetrenone specifications and serves as a tool for batch-level quality control.
Menatetrenone CoA mostly includes findings from lab analyses of a specific batch. For each Menatetrenone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Menatetrenone may be tested according to a variety of international standards, such as European Pharmacopoeia (Menatetrenone EP), Menatetrenone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Menatetrenone USP).
