

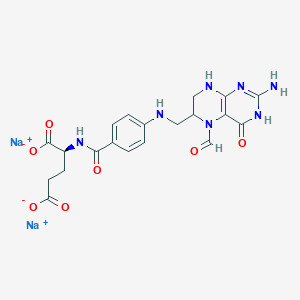

1. Sodium Folinate
2. Oncofolic
3. Folinic Acid Disodium
4. Disodium Folinate
5. 4mxu9ljs4q
6. 163254-40-8
7. Unii-4mxu9ljs4q
8. Chembl3707260
9. Sodium Folinate [mart.]
10. Sodium Folinate [who-dd]
11. Q27260173
12. L-glutamic Acid, N-(4-(((2-amino-5-formyl-1,4,5,6,7,8-hexahydro-4-oxo-6-pteridinyl)methyl)amino)benzoyl)-, Disodium Salt
| Molecular Weight | 517.4 g/mol |
|---|---|
| Molecular Formula | C20H21N7Na2O7 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 7 |
| Exact Mass | 517.12978460 g/mol |
| Monoisotopic Mass | 517.12978460 g/mol |
| Topological Polar Surface Area | 221 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 900 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
V - Various
V03 - All other therapeutic products
V03A - All other therapeutic products
V03AF - Detoxifying agents for antineoplastic treatment
V03AF06 - Sodium folinate