
Synopsis
0
VMF
0
Weekly News Recap #Phispers
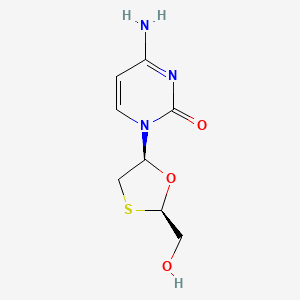

1. 2',3' Dideoxy 3' Thiacytidine
2. 2',3'-dideoxy-3'-thiacytidine
3. 2(1h)-pyrimidinone, 4-amino-1-(2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-, (2r-cis)-
4. 3tc Lamivudine
5. Bch 189
6. Bch-189
7. Bch189
8. Epivir
9. Gr 109714x
10. Gr-109714x
11. Gr109714x
12. Lamivudine, (+)-cis-
13. Lamivudine, (+-)-trans-
14. Lamivudine, (2s-cis)-isomer
15. Lamivudine, 3tc
1. 134678-17-4
2. Epivir
3. Zeffix
4. Heptovir
5. Epivir-hbv
6. 136891-12-8
7. 3tc
8. Bch-189
9. Heptodin
10. 4-amino-1-((2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)pyrimidin-2(1h)-one
11. (-)-2'-deoxy-3'-thiacytidine
12. Virolam
13. Gr-109714x
14. 3'-thia-2',3'-dideoxycytidine
15. Gr109714x
16. (-)-bch-189
17. Beta-l-2',3'-dideoxy-3'-thiacytidine
18. Beta-l-3'-thia-2',3'-dideoxycytidine
19. 2',3'-dideoxy-3'-thiacytidine
20. Gr 109714x
21. Lamivudine Teva
22. 4-amino-1-[(2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one
23. (-)ngpb-21
24. Bch 189, (-)-
25. (-)-1-((2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)cytosine
26. 4-amino-1-(cis-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)pyrimidin-2(1h)-one
27. 4-amino-1-[(2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-1,2-dihydropyrimidin-2-one
28. Hepitec
29. 4-amino-1-[(2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2(1h)-one
30. 4-amino-1-[(2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-2(1h)-pyrimidinone
31. Chebi:63577
32. (-)-1-[(2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine
33. 4-amino-1-((2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-2(1h)-pyrimidinone
34. 2(1h)-pyrimidinone, 4-amino-1-(2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-, (2r-cis)-
35. 2t8q726o95
36. Nsc-760061
37. Heptivir
38. Lamivir
39. Zefix
40. Bch 189
41. Lamivudine [usan:ban:inn]
42. (-)-(2'r,5's)-1-[2'-hydroxymethyl-5'-(1,3-oxathiolanyl)]cytosine
43. 2(1h)-pyrimidinone, 4-amino-1-((2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-
44. 2(1h)-pyrimidinone, 4-amino-1-[(2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-
45. Epivir(tm)
46. Hha & Lamivudine
47. Lamivudine & Gna
48. Smr000466319
49. Epivir (tn)
50. (-)-bch 189
51. Dthc
52. Chembl141
53. Hsdb 7155
54. Drg-0126
55. 3tc And Nv-01
56. (-)-sddc
57. Lamivudeine
58. Lamivudinum
59. Lamivudine (jan/usp/inn)
60. 3tc & Gna
61. 3tc & Sst
62. Hha & 3tc
63. (+/-)-sddc
64. Ccris 9274
65. Nsc620753
66. Unii-2t8q726o95
67. Bch-790
68. Lamivudine [usan:usp:inn:ban]
69. Rac-cis-lamivudine
70. Ncgc00159341-04
71. Lamivudine- Bio-x
72. Gg-714
73. Mfcd00869739
74. Lamivudine (epivir)
75. Cpd000466319
76. Lamivudine [mi]
77. (+/-)-3tc
78. (+/-)-bch-189
79. Lamivudine [inn]
80. Lamivudine [jan]
81. Lamivudine [hsdb]
82. Lamivudine [usan]
83. Lamivudine [vandf]
84. Lamivudine [mart.]
85. Lamivudine [usp-rs]
86. Lamivudine [who-dd]
87. Lamivudine [who-ip]
88. Mls000759424
89. Mls001424097
90. Mls006011910
91. Bidd:gt0033
92. Lamivudine [ema Epar]
93. Schembl109675
94. Amy384
95. Dtxsid7023194
96. Lamivudine [ep Impurity]
97. Lamivudine [orange Book]
98. Zinc12346
99. Lamivudine [ep Monograph]
100. Kivexa Component Lamivudine
101. Hms2051d21
102. Hms3259f08
103. Hms3713c16
104. Lamivudine [usp Monograph]
105. Epzicom Component Lamivudine
106. Lamivudinum [who-ip Latin]
107. Temixys Component Lamivudine
108. Triumeq Component Lamivudine
109. Combivir Component Lamivudine
110. Hy-b0250
111. Lamivudine Teva Pharma B.v.
112. Trizivir Component Lamivudine
113. Bbl033871
114. Bdbm50366817
115. Delstrigo Component Lamivudine
116. Mfcd00870542
117. S1706
118. Stk801940
119. Lamivudine & Tnf-alpha & Ifn-gamma
120. Telura Component Of Lamivudine
121. Akos005622556
122. Akos015854841
123. Lamivudine Component Of Epzicom
124. Lamivudine Component Of Temixys
125. Lamivudine Component Of Triumeq
126. Lamivudine, >=98% (hplc), Powder
127. (-)-bch189
128. Ac-1416
129. Ccg-100984
130. Db00709
131. Ks-1073
132. Lamivudine Component Of Combivir
133. Lamivudine Component Of Dutrebis
134. Lamivudine Component Of Trizivir
135. Nc00234
136. Nc00705
137. Nsc 760061
138. Lamivudine Component Of Delstrigo
139. Ncgc00159341-05
140. Ncgc00159341-18
141. Ncgc00159341-20
142. Bl164607
143. Emtricitabine Impurity C [who-ip]
144. L0217
145. Rac-cis-lamivudine ((2rs,5sr)-lamivudine)
146. Sw197614-3
147. C07065
148. D00353
149. P17147
150. Ab00639995-06
151. Ab00639995-08
152. Ab00639995_09
153. Lamivudeine 100 Microg/ml In Acetonitrile:water
154. 678l174
155. Q422631
156. Sr-01000759420
157. J-700183
158. Q-201275
159. Sr-01000759420-5
160. Lamivudine/zidovudine Teva Component Lamivudine
161. Lamivudine Component Of Lamivudine/zidovudine Teva
162. Lamivudine, British Pharmacopoeia (bp) Reference Standard
163. Lamivudine, European Pharmacopoeia (ep) Reference Standard
164. (2r,5s)-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine
165. .beta.-l-(-)-2',3'-dideoxy-3'-thiacytidine & Sho-saiko-to
166. Lamivudine, United States Pharmacopeia (usp) Reference Standard
167. (+/-) (cis)-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine
168. (-)-l-2',3'-dideoxy-3'-thiacytidine; Lamivudine; Epivir
169. Lamivudine, 1.0 Mg/ml In Methanol, Certified Reference Material
170. Lamivudine, Pharmaceutical Secondary Standard; Certified Reference Material
171. (+/-)-(cis)-4-amino-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(1h)-pyrimidin-2-one
172. 2(1h)-pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl], (+/-) (cis)
173. 4-amino-1-[(2r,5s)-2-(hydroxymethyl)-[1,3]-oxathiolan-5-yl]-(1h)-pyrimidin-2-one
174. 4-amino-1-[(2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-(1h)-pyrimidin-2-one
175. Cis(+/-)-4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-2(1h)-pyrimidinone
176. Lamivudine For System Suitability 1, European Pharmacopoeia (ep) Reference Standard
177. Lamivudine For System Suitability 2, European Pharmacopoeia (ep) Reference Standard
178. 1117764-41-6
179. 2(1h)-pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl], (-)(2r,5s) & Galanthus Nivalis Agglutinin (gna)
180. 2(1h)-pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl], (-)(2r,5s) & Hippeastrum Hybrid Agglutinin( Hha)
181. 4-amino-1-((2r,5s)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)pyrimidin-2(1h)-one [who-ip]
| Molecular Weight | 229.26 g/mol |
|---|---|
| Molecular Formula | C8H11N3O3S |
| XLogP3 | -0.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 229.05211239 g/mol |
| Monoisotopic Mass | 229.05211239 g/mol |
| Topological Polar Surface Area | 113 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 331 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Epivir |
| Drug Label | EPIVIR-HBV is a brand name for lamivudine, a synthetic nucleoside analogue with activity against hepatitis B virus (HBV) and HIV. Lamivudine was initially developed for the treatment of HIV infection as EPIVIR. Please see the complete prescribing inf... |
| Active Ingredient | Lamivudine |
| Dosage Form | Tablet; Solution |
| Route | Oral |
| Strength | 300mg; 150mg; 10mg/ml |
| Market Status | Prescription |
| Company | Viiv Hlthcare |
| 2 of 6 | |
|---|---|
| Drug Name | Epivir-hbv |
| PubMed Health | Lamivudine (By mouth) |
| Drug Classes | Antiretroviral Agent, Antiviral |
| Drug Label | EPIVIR-HBV is a synthetic nucleoside analogue with activity against HBV. The chemical name of lamivudine is (2R,cis)-4-amino-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(1H)-pyrimidin-2-one. Lamivudine is the (-)enantiomer of a dideoxy analogue of cytidi... |
| Active Ingredient | Lamivudine |
| Dosage Form | Tablet; Solution |
| Route | Oral |
| Strength | 5mg/ml; 100mg |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 3 of 6 | |
|---|---|
| Drug Name | Lamivudine |
| PubMed Health | Lamivudine (By mouth) |
| Drug Classes | Antiretroviral Agent, Antiviral |
| Drug Label | EPIVIR-HBV is a brand name for lamivudine, a synthetic nucleoside analogue with activity against hepatitis B virus (HBV) and HIV. Lamivudine was initially developed for the treatment of HIV infection as EPIVIR. Please see the complete prescribing inf... |
| Active Ingredient | Lamivudine |
| Dosage Form | Tablet; Solution |
| Route | oral; Oral |
| Strength | 150mg; 10mg/ml; 300mg; 100mg |
| Market Status | Tentative Approval; Prescription |
| Company | Ranbaxy; Hetero Labs Ltd V; Macleods Pharma; Apotex; Aurobindo; Hetero Labs Ltd Iii; Alkem Labs; Aurobindo Pharma; Invagen Pharms; Cipla; Matrix Labs; Teva Pharms; Strides Arcolab; Micro Labs |
| 4 of 6 | |
|---|---|
| Drug Name | Epivir |
| Drug Label | EPIVIR-HBV is a brand name for lamivudine, a synthetic nucleoside analogue with activity against hepatitis B virus (HBV) and HIV. Lamivudine was initially developed for the treatment of HIV infection as EPIVIR. Please see the complete prescribing inf... |
| Active Ingredient | Lamivudine |
| Dosage Form | Tablet; Solution |
| Route | Oral |
| Strength | 300mg; 150mg; 10mg/ml |
| Market Status | Prescription |
| Company | Viiv Hlthcare |
| 5 of 6 | |
|---|---|
| Drug Name | Epivir-hbv |
| PubMed Health | Lamivudine (By mouth) |
| Drug Classes | Antiretroviral Agent, Antiviral |
| Drug Label | EPIVIR-HBV is a synthetic nucleoside analogue with activity against HBV. The chemical name of lamivudine is (2R,cis)-4-amino-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(1H)-pyrimidin-2-one. Lamivudine is the (-)enantiomer of a dideoxy analogue of cytidi... |
| Active Ingredient | Lamivudine |
| Dosage Form | Tablet; Solution |
| Route | Oral |
| Strength | 5mg/ml; 100mg |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 6 of 6 | |
|---|---|
| Drug Name | Lamivudine |
| PubMed Health | Lamivudine (By mouth) |
| Drug Classes | Antiretroviral Agent, Antiviral |
| Drug Label | EPIVIR-HBV is a brand name for lamivudine, a synthetic nucleoside analogue with activity against hepatitis B virus (HBV) and HIV. Lamivudine was initially developed for the treatment of HIV infection as EPIVIR. Please see the complete prescribing inf... |
| Active Ingredient | Lamivudine |
| Dosage Form | Tablet; Solution |
| Route | oral; Oral |
| Strength | 150mg; 10mg/ml; 300mg; 100mg |
| Market Status | Tentative Approval; Prescription |
| Company | Ranbaxy; Hetero Labs Ltd V; Macleods Pharma; Apotex; Aurobindo; Hetero Labs Ltd Iii; Alkem Labs; Aurobindo Pharma; Invagen Pharms; Cipla; Matrix Labs; Teva Pharms; Strides Arcolab; Micro Labs |
Lamivudine is indicated in the treatment of chronic hepatitis B associated with evidence of hepatitis B viral replication and active liver inflammation. This use is based on 1-year histologic and serologic responses in patients with compensated chronic hepatitis B. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1646
Lamivudine is indicated, in combination with zidovudine or other antiretroviral agents, in the treatment of HIV infection or AIDS when therapy is warranted based on clinical and/or immunological evidence of disease progression. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1646
Lamivudine may be used prophylactically in health care workers at risk of acquiring HIV infection after occupational exposure to the virus. It is being used in combination with zidovudine and, in some cases, a protease inhibitor. /NOT included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1646
The safety, pharmacokinetics, and antiretroviral activity of lamivudine alone and in combination with zidovudine was studied in pregnant women infected with human immunodeficiency virus type 1 (HIV-1) and their neonates. Women received the drugs orally from week 38 of pregnancy to 1 week after delivery. Neonate therapy began 12 h after delivery and continued for 1 week. Both treatment regimens were well-tolerated in women and newborns. Lamivudine and zidovudine pharmacokinetics in pregnant women were similar to those in nonpregnant adults. Lamivudine and zidovudine freely crossed the placenta and were secreted in breast milk. Neonatal lamivudine clearance was about half that in pediatric patients; zidovudine clearance was consistent with previous reports. HIV-1 RNA could be quantified in 17 of the 20 women. At the onset of labor/delivery, mean virus load had decreased by approximately 1.5 log10 copies/mL in both treatment cohorts. Although not definitive for HIV-1 infection status, all neonates had HIV-1 RNA levels below the limit of quantification at birth and at ages 1 and 2 weeks.
PMID:9780252 Moodley J et al; J Infect Dis 178 (5): 1327-33 (1998)
Safety and efficacy of lamivudine in the treatment of chronic hepatitis B have not been established in patients with decompensated liver disease or organ transplants; pediatric patients; or patients dually infected with hepatitis B and hepatitis C, hepatitis delta, or HIV; or for a treatment period greater than 1 year.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1646
Although lamivudine generally is well tolerated, serious adverse effects such as peripheral neuropathy, pancreatitis, and lactic acidosis and severe hepatomegaly with steatosis have been reported. Adverse GI effects are the most common adverse effects in patients receiving lamivudine alone or in conjunction with zidovudine. Information on adverse effects of lamivudine has been obtained from clinical studies in HIV-infected adults who received the drug in conjunction with other antiretroviral agents ... . In addition, information on adverse effects of lamivudine in patients with compensated chronic hepatitis B virus (HBV) infection has been obtained from 3 placebo-controlled studies where the drug was used alone for up to 68 weeks.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 668
Peripheral neuropathy, the major dose-limiting toxicity associated with zalcitabine therapy, has been reported in adults receiving lamivudine, but has rarely resulted in interruption or discontinuance of therapy. In clinical studies... in HIV-infected adults receiving lamivudine in conjunction with zidovudine, neuropathy was reported in 12% of the patients. Paresthesia, weakness, and peripheral neuropathy have been reported in patients receiving lamivudine during postmarketing surveillance. ... In clinical studies in adults who received lamivudine for the treatment of chronic /hepatitis B virus/ (HBV) infection, malaise, fatigue, and headache were reported in 24, 24, and 21% of patients, respectively.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 668
In HIV-infected adults receiving lamivudine in conjunction with zidovudine, headache, malaise, fatigue, insomnia and other sleep disorders, dizziness, and depressive disorders were reported in 35, 27, 27, 11, 10, and 9%, respectively.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 668
For more Drug Warnings (Complete) data for LAMIVUDINE (20 total), please visit the HSDB record page.
For the treatment of HIV infection and chronic hepatitis B (HBV).
FDA Label
Epivir is indicated as part of antiretroviral combination therapy for the treatment of human-immunodeficiency-virus (HIV)-infected adults and children.
Lamivudine Teva Pharma B. V. is indicated as part of antiretroviral combination therapy for the treatment of human-immunodeficiency-virus (HIV)-infected adults and children.
Zeffix is indicated for the treatment of chronic hepatitis B in adults with:
- compensated liver disease with evidence of active viral replication, persistently elevated serum alanine aminotransferase (ALT) levels and histological evidence of active liver inflammation and / or fibrosis. Initiation of lamivudine treatment should only be considered when the use of an alternative antiviral agent with a higher genetic barrier is not available or appropriate;
- decompensated liver disease in combination with a second agent without cross-resistance to lamivudine.
Lamivudine Teva is indicated for the treatment of chronic hepatitis B in adults with:
- compensated liver disease with evidence of active viral replication, persistently elevated serum alanine aminotransferase (ALT) levels and histological evidence of active liver inflammation and / or fibrosis. Initiation of lamivudine treatment should only be considered when the use of an alternative antiviral agent with a higher genetic barrier is not available or appropriate (see in section 5. 1).
Lamivudine is a nucleoside reverse transcriptase inhibitor (NRTI) with activity against Human Immunodeficiency Virus Type 1 (HIV-1) and hepatitis B (HBV) to disrupt viral DNA synthesis. When phosphorylated, lamivudine can form active metabolites that compete for incorporation into viral DNA. Via DNA incorporation, lamivudine metabolites competitively inhibit the activity of the HIV reverse transcriptase enzyme and act as a chain terminator of DNA synthesis. Due to the lack of a 3'-OH group, incorporated nucleoside analogues prevent the formation of a 5' to 3' phosphodiester linkage that is essential for DNA chain elongation.
Anti-HIV Agents
Agents used to treat AIDS and/or stop the spread of the HIV infection. These do not include drugs used to treat symptoms or opportunistic infections associated with AIDS. (See all compounds classified as Anti-HIV Agents.)
Reverse Transcriptase Inhibitors
Inhibitors of reverse transcriptase (RNA-DIRECTED DNA POLYMERASE), an enzyme that synthesizes DNA on an RNA template. (See all compounds classified as Reverse Transcriptase Inhibitors.)
J05AF05
J05AF05
J05AF05
J05AF05
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AF - Nucleoside and nucleotide reverse transcriptase inhibitors
J05AF05 - Lamivudine
Absorption
Lamivudine was rapidly absorbed after oral administration in HIV-infected patients. Absolute bioavailability in 12 adult patients was 86% 16% (mean SD) for the 150-mg tablet and 87% 13% for the oral solution. The peak serum lamivudine concentration (Cmax) was 1.5 0.5 mcg/mL when an oral dose of 2 mg/kg twice a day was given to HIV-1 patients. When given with food, absorption is slower, compared to the fasted state.
Route of Elimination
The majority of lamivudine is eliminated unchanged in urine by active organic cationic secretion. 5.2% 1.4% (mean SD) of the dose was excreted as the trans-sulfoxide metabolite in the urine. Lamivudine is excreted in human breast milk and into the milk of lactating rats.
Volume of Distribution
Apparent volume of distribution, IV administration = 1.3 0.4 L/kg. Volume of distribution was independent of dose and did not correlate with body weight.
Clearance
Renal clearance = 199.7 56.9 mL/min [300 mg oral dose, healthy subjects]
Renal clearance = 280.4 75.2 mL/min [single IV dose, HIV-1-infected patients]
Total clearance = 398.5 69.1 mL/min [HIV-1-infected patients]
Lamivudine crosses the placenta and has been detected in the fetal circulation.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1359
Lamivudine has high oral bioavailability with or without food and reaches peak plasma levels within approximately 1 hour.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1359
Metabolism of lamivudine is a minor route of elimination. In man, the only known metabolite of lamivudine is the trans-sulfoxide metabolite. This biotransformation is catalyzed by sulfotransferases.
5 to 7 hours (healthy or HBV-infected patients)
Lamivudine is a synthetic nucleoside analogue and is phosphorylated intracellularly to its active 5'-triphosphate metabolite, lamivudine triphosphate (L-TP). This nucleoside analogue is incorporated into viral DNA by HIV reverse transcriptase and HBV polymerase, resulting in DNA chain termination.
Lamivudine enters cells by passive diffusion and is phosphorylated to its active metabolite, lamivudine triphosphate. Lamivudine triphosphate competes with deoxycytidine triphosphate for binding to reverse transcriptase, and incorporation into DNA results in chain termination. Lamivudine has very low affinity for human alpha and omega DNA polymerases, moderate affinity for beta DNA polymerase, and higher affinity for gamma DNA polymerase.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1358

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
57
PharmaCompass offers a list of Lamivudine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Lamivudine manufacturer or Lamivudine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Lamivudine manufacturer or Lamivudine supplier.
PharmaCompass also assists you with knowing the Lamivudine API Price utilized in the formulation of products. Lamivudine API Price is not always fixed or binding as the Lamivudine Price is obtained through a variety of data sources. The Lamivudine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Lamivudine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Lamivudine, including repackagers and relabelers. The FDA regulates Lamivudine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Lamivudine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Lamivudine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Lamivudine supplier is an individual or a company that provides Lamivudine active pharmaceutical ingredient (API) or Lamivudine finished formulations upon request. The Lamivudine suppliers may include Lamivudine API manufacturers, exporters, distributors and traders.
click here to find a list of Lamivudine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Lamivudine DMF (Drug Master File) is a document detailing the whole manufacturing process of Lamivudine active pharmaceutical ingredient (API) in detail. Different forms of Lamivudine DMFs exist exist since differing nations have different regulations, such as Lamivudine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Lamivudine DMF submitted to regulatory agencies in the US is known as a USDMF. Lamivudine USDMF includes data on Lamivudine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Lamivudine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Lamivudine suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Lamivudine Drug Master File in Japan (Lamivudine JDMF) empowers Lamivudine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Lamivudine JDMF during the approval evaluation for pharmaceutical products. At the time of Lamivudine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Lamivudine suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Lamivudine Drug Master File in Korea (Lamivudine KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Lamivudine. The MFDS reviews the Lamivudine KDMF as part of the drug registration process and uses the information provided in the Lamivudine KDMF to evaluate the safety and efficacy of the drug.
After submitting a Lamivudine KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Lamivudine API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Lamivudine suppliers with KDMF on PharmaCompass.
A Lamivudine CEP of the European Pharmacopoeia monograph is often referred to as a Lamivudine Certificate of Suitability (COS). The purpose of a Lamivudine CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Lamivudine EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Lamivudine to their clients by showing that a Lamivudine CEP has been issued for it. The manufacturer submits a Lamivudine CEP (COS) as part of the market authorization procedure, and it takes on the role of a Lamivudine CEP holder for the record. Additionally, the data presented in the Lamivudine CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Lamivudine DMF.
A Lamivudine CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Lamivudine CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Lamivudine suppliers with CEP (COS) on PharmaCompass.
A Lamivudine written confirmation (Lamivudine WC) is an official document issued by a regulatory agency to a Lamivudine manufacturer, verifying that the manufacturing facility of a Lamivudine active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Lamivudine APIs or Lamivudine finished pharmaceutical products to another nation, regulatory agencies frequently require a Lamivudine WC (written confirmation) as part of the regulatory process.
click here to find a list of Lamivudine suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Lamivudine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Lamivudine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Lamivudine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Lamivudine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Lamivudine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Lamivudine suppliers with NDC on PharmaCompass.
Lamivudine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Lamivudine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Lamivudine GMP manufacturer or Lamivudine GMP API supplier for your needs.
A Lamivudine CoA (Certificate of Analysis) is a formal document that attests to Lamivudine's compliance with Lamivudine specifications and serves as a tool for batch-level quality control.
Lamivudine CoA mostly includes findings from lab analyses of a specific batch. For each Lamivudine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Lamivudine may be tested according to a variety of international standards, such as European Pharmacopoeia (Lamivudine EP), Lamivudine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Lamivudine USP).

