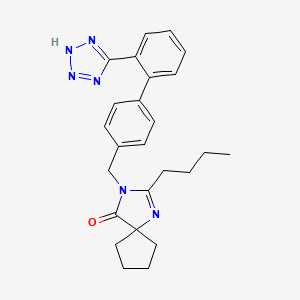

1. 2-n-butyl-3-((2'-(1h-tetrazol-5-yl)biphenyl-4-yl)methyl)-1,3-diazaspiro(4,4)non-1-en-4-one
2. Aprovel
3. Avapro
4. Bms 186295
5. Bms-186295
6. Karvea
7. Sr 47436
8. Sr-47436
9. Sr47436
1. 138402-11-6
2. Avapro
3. Aprovel
4. Karvea
5. Sr-47436
6. Bms-186295
7. Irbesartan Bms
8. Sr 47436
9. Bms 186295
10. Irbesartan Zentiva
11. Irbesartan D4
12. Sarbevel
13. Irbesartan Teva
14. Irbesartan Free Base
15. Nsc-758696
16. Chembl1513
17. 2-butyl-3-[[4-[2-(2h-tetrazol-5-yl)phenyl]phenyl]methyl]-1,3-diazaspiro[4.4]non-1-en-4-one
18. 2-butyl-3-{[2'-(1h-tetrazol-5-yl)biphenyl-4-yl]methyl}-1,3-diazaspiro[4.4]non-1-en-4-one
19. 8-butyl-7-[[4-[2-(2h-tetrazol-5-yl)phenyl]phenyl]methyl]-7,9-diazaspiro[4.4]non-8-en-6-one
20. Chebi:5959
21. J0e2756z7n
22. 138402-11-6 (free Base)
23. 2-butyl-3-(p-(o-1h-tetrazol-5-ylphenyl)benzyl)-1,3-diazaspiro(4.4)non-1-en-4-one
24. 1,3-diazaspiro(4.4)non-1-en-4-one, 2-butyl-3-((2'-(1h-tetrazol-5-yl)(1,1'-biphenyl)-4-yl)methyl)-
25. Ncgc00095122-01
26. Dsstox_cid_3169
27. Dsstox_rid_76900
28. Dsstox_gsid_23169
29. 1,3-diazaspiro[4.4]non-1-en-4-one, 2-butyl-3-[[2'-(2h-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-
30. 2-butyl-3-{[2'-(1h-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl}-1,3-diazaspiro[4.4]non-1-en-4-one
31. Irbetan
32. Irbesartan Krka
33. [3h]irbesartan
34. 3-((2'-(2h-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-1,3-diazaspiro[4.4]non-1-en-4-one
35. Irbesartan Winthrop
36. Smr000466306
37. Avapro (tn)
38. Cas-138402-11-6
39. Unii-j0e2756z7n
40. 3-((2'-(1h-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-2-butyl-1,3-diazaspiro[4.4]non-1-en-4-one
41. Hsdb 8215
42. Irbesartan [usan:usp:inn:ban]
43. Irbesartan ,(s)
44. 2-butyl-3-[[4-[2-(1h-tetrazol-5-yl)phenyl]phenyl]methyl]-1,3-diazaspiro[4.4]non-1-en-4-one
45. 3-((2'-(1h-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-1,3-diazaspiro[4.4]non-1-en-4-one
46. Irbesartan- Bio-x
47. Irbesartan (avapro)
48. Mfcd00864464
49. Spectrum_001751
50. Irbesartan [mi]
51. Irbesartan [inn]
52. Irbesartan [jan]
53. Spectrum2_001675
54. Spectrum3_000994
55. Spectrum4_001122
56. Spectrum5_001288
57. Irbesartan [usan]
58. Irbesartan [vandf]
59. Irbesartan [mart.]
60. Schembl4246
61. Irbesartan [usp-rs]
62. Irbesartan [who-dd]
63. Bspbio_002687
64. Gtpl589
65. Kbiogr_001603
66. Kbioss_002231
67. Mls000759408
68. Mls001424099
69. Bidd:gt0347
70. Irbesartan [ema Epar]
71. Spectrum1504259
72. Spbio_001889
73. Gtpl6908
74. Irbesartan (jp17/usp/inn)
75. Dtxsid0023169
76. Irbesartan [ep Impurity]
77. Irbesartan [orange Book]
78. Kbio2_002231
79. Kbio2_004799
80. Kbio2_007367
81. Kbio3_001907
82. Irbesartan [ep Monograph]
83. Irbesartan [usp Impurity]
84. Bcpp000202
85. Hms1922j05
86. Hms2051l08
87. Hms2093e16
88. Hms2232f23
89. Hms3370b06
90. Hms3393l08
91. Hms3715l04
92. Irbesartan [usp Monograph]
93. Pharmakon1600-01504259
94. Avalide Component Irbesartan
95. Bcp02004
96. Hy-b0202
97. Zinc3872931
98. Tox21_111433
99. Ac-537
100. Bdbm50042235
101. Ccg-39091
102. Coaprovel Component Irbesartan
103. Nsc758696
104. Stk645362
105. Akos005576396
106. Akos015895353
107. Ifirmacombi Component Irbesartan
108. Irbesartan Component Of Avalide
109. Irbesartan, >=98% (hplc), Powder
110. Tox21_111433_1
111. Ab07472
112. Am90289
113. Bcp9000792
114. Ccg-101012
115. Db01029
116. Ks-1151
117. Nc00262
118. Nsc 758696
119. 2-butyl-3-[2'-(1h-tetrazol-5-yl)biphenyl-4-ylmethyl]1,3-diaza-spiro[4.4]non-1-en-4-one
120. Irbesartan Component Of Coaprovel
121. Irbesartan Component Of Karvezide
122. Ncgc00095122-02
123. Ncgc00095122-03
124. Ncgc00095122-04
125. Ncgc00095122-05
126. Ncgc00095122-14
127. 2-butyl-3-[[2'-(2h-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-1,3-diazaspiro[4.4]non-1-en-4-one
128. Bi164582
129. Irbesartan Component Of Ifirmacombi
130. Sbi-0206769.p001
131. Ft-0601598
132. Ft-0670413
133. I0859
134. S1507
135. Sr-47436;bms-186295
136. C07469
137. D00523
138. Ab00639954-06
139. Ab00639954_07
140. Ab00639954_08
141. 402i116
142. A807387
143. L000319
144. Q947266
145. Sr-05000001997
146. Q-201249
147. Sr-05000001997-1
148. Brd-k60038276-001-02-5
149. Brd-k60038276-001-03-3
150. Ibersartan/hydrochlorothiazide Component Ibersartan
151. Irbesartan, European Pharmacopoeia (ep) Reference Standard
152. Ibersartan/hydrochlorothiazide Teva Component Ibersartan
153. Irbesartan, United States Pharmacopeia (usp) Reference Standard
154. Ibersartan Component Of Ibersartan/hydrochlorothiazide Teva
155. Ibersartan Component Of Ibersartan/hydrochlorothiazide Zentiva
156. Ibersartan/hydrochlorothiazide Zentiva Component Ibersartan
157. Irbesartan, Pharmaceutical Secondary Standard; Certified Reference Material
158. 2-(n-butyl)-3-[[2'-(tetrazol-5-yl)biphenyl-4-yl]methyl]-1,3-diazaspiro[4.4]non-1-en-4-one
159. 2-butyl-3-({4-[2-(2h-1,2,3,4-tetrazol-5-yl)phenyl]phenyl}methyl)-1,3-diazaspiro[4.4]non-1-en-4-one
160. 2-butyl-3-[2'-(1h-tetrazol-5-yl)-biphenyl-4-ylmethyl]-1,3-diaza-spiro[4.4]non-1-en-4-one
161. 2-butyl-3-{[2''-(1h-tetrazol-5-yl)[1,1''-biphenyl]-4-yl]methyl}-1,3-diazaspiro[4.4]non-1-en-4-one
162. 2-butyl-3-{[2''-(1h-tetrazol-5-yl)biphenyl-4-yl]methyl}-1,3-diazaspiro[4.4]non-1-en-4-one
163. 3-((2'-(1h-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)-methyl)-2-butyl-1,3-diazaspiro[4.4]non-1-en-4-one
164. 8-butyl-7-[[4-[2-(2h-1,2,3,4-tetrazol-5-yl)phenyl]phenyl]methyl]-7,9-diazaspiro[4.4]non-8-en-6-one
| Molecular Weight | 428.5 g/mol |
|---|---|
| Molecular Formula | C25H28N6O |
| XLogP3 | 4.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 7 |
| Exact Mass | 428.23245954 g/mol |
| Monoisotopic Mass | 428.23245954 g/mol |
| Topological Polar Surface Area | 87.1 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 682 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Avapro |
| PubMed Health | Irbesartan (By mouth) |
| Drug Classes | Cardiovascular Agent, Renal Protective Agent |
| Drug Label | AVAPRO* (irbesartan) is an angiotensin II receptor (AT1 subtype) antagonist.*Registered trademark Irbesartan is a non-peptide compound, chemically described as a 2-butyl-3-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]-1,3-diazaspiro[4.4]non-1-en-4-one.Its e... |
| Active Ingredient | Irbesartan |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 300mg; 75mg; 150mg |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
| 2 of 4 | |
|---|---|
| Drug Name | Irbesartan |
| PubMed Health | Irbesartan (By mouth) |
| Drug Classes | Cardiovascular Agent, Renal Protective Agent |
| Drug Label | Irbesartan USP is an angiotensin II receptor (AT1 subtype) antagonist.Irbesartan USP is a non-peptide compound, chemically described as a 2-butyl-3-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]-1,3-diazaspiro[4.4]non-1-en-4-one.Its empirical formula is C25H28... |
| Active Ingredient | Irbesartan |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 150mg; 300mg; 75mg |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharms; Hetero Labs Ltd V; Apotex; Alembic Pharms; Aurobindo Pharma; Lupin; Sandoz; Prinston; Cipla; Roxane; Watson Labs; Teva Pharms; Macleods Pharms; Zydus Pharms Usa; Dr Reddys Labs; Unichem Labs |
| 3 of 4 | |
|---|---|
| Drug Name | Avapro |
| PubMed Health | Irbesartan (By mouth) |
| Drug Classes | Cardiovascular Agent, Renal Protective Agent |
| Drug Label | AVAPRO* (irbesartan) is an angiotensin II receptor (AT1 subtype) antagonist.*Registered trademark Irbesartan is a non-peptide compound, chemically described as a 2-butyl-3-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]-1,3-diazaspiro[4.4]non-1-en-4-one.Its e... |
| Active Ingredient | Irbesartan |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 300mg; 75mg; 150mg |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
| 4 of 4 | |
|---|---|
| Drug Name | Irbesartan |
| PubMed Health | Irbesartan (By mouth) |
| Drug Classes | Cardiovascular Agent, Renal Protective Agent |
| Drug Label | Irbesartan USP is an angiotensin II receptor (AT1 subtype) antagonist.Irbesartan USP is a non-peptide compound, chemically described as a 2-butyl-3-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]-1,3-diazaspiro[4.4]non-1-en-4-one.Its empirical formula is C25H28... |
| Active Ingredient | Irbesartan |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 150mg; 300mg; 75mg |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharms; Hetero Labs Ltd V; Apotex; Alembic Pharms; Aurobindo Pharma; Lupin; Sandoz; Prinston; Cipla; Roxane; Watson Labs; Teva Pharms; Macleods Pharms; Zydus Pharms Usa; Dr Reddys Labs; Unichem Labs |
Angiotensin II Type 1 Receptor Blockers; Antihypertensive Agents
National Library of Medicine's Medical Subject Headings. Irbesartan. Online file (MeSH, 2014). Available from, as of September 2, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Avapro (irbesartan) is indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive agents. /Included in US product label/
NIH; DailyMed. Current Medication Information for Avapro (Irbesartan) Tablet (Revised: September 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b7f1a9ef-c7bb-465c-e4d7-a41395205cad
Avapro is indicated for the treatment of diabetic nephropathy with an elevated serum creatinine and proteinuria (>300 mg/day) in patients with type 2 diabetes and hypertension. In this population, Avapro reduces the rate of progression of nephropathy as measured by the occurrence of doubling of serum creatinine or end-stage renal disease (need for dialysis or renal transplantation). /Included in US product label/
NIH; DailyMed. Current Medication Information for Avapro (Irbesartan) Tablet (Revised: September 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b7f1a9ef-c7bb-465c-e4d7-a41395205cad
Angiotensin II receptor antagonists /including irbesartan/ have been used in the management of congestive heart failure. /NOT included in US product label/
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2063
/BOXED WARNING/ WARNING: FETAL TOXICITY. When pregnancy is detected, discontinue Avapro as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus.
NIH; DailyMed. Current Medication Information for Avapro (Irbesartan) Tablet (Revised: September 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b7f1a9ef-c7bb-465c-e4d7-a41395205cad
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Avapro as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimesters of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus. In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue Avapro, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury.
NIH; DailyMed. Current Medication Information for Avapro (Irbesartan) Tablet (Revised: September 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b7f1a9ef-c7bb-465c-e4d7-a41395205cad
Neonates with a history of in utero exposure to Avapro: If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function.
NIH; DailyMed. Current Medication Information for Avapro (Irbesartan) Tablet (Revised: September 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b7f1a9ef-c7bb-465c-e4d7-a41395205cad
FDA Pregnancy Risk Category: D /POSITIVE EVIDENCE OF RISK. Studies in humans, or investigational or post-marketing data, have demonstrated fetal risk. Nevertheless, potential benefits from the use of the drug may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective./
NIH; DailyMed. Current Medication Information for Avapro (Irbesartan) Tablet (Revised: September 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b7f1a9ef-c7bb-465c-e4d7-a41395205cad
For more Drug Warnings (Complete) data for Irbesartan (16 total), please visit the HSDB record page.
Irbesartan is indicated to treat hypertension and diabetic nephropathy in hypertensive patients with type 2 diabetes, elevated serum creatinine, and proteinuria. A combination product with hydrochlorothiazide is indicated for hypertension in patients with uncontrolled hypertension with monotherapy or first line in patients not expected to be well controlled with monotherapy.
FDA Label
Treatment of essential hypertension.
Treatment of renal disease in patients with hypertension and type-2 diabetes mellitus as part of an antihypertensive medicinal product regimen.
Treatment of essential hypertension.
Treatment of renal disease in patients with hypertension and type 2 diabetes mellitus as part of an antihypertensive medicinal product regimen.
Treatment of essential hypertension.
Treatment of renal disease in patients with hypertension and type-2 diabetes mellitus as part of an antihypertensive medicinal-product regimen.
Treatment of essential hypertension.
Treatment of renal disease in patients with hypertension and type 2 diabetes mellitus as part of an antihypertensive medicinal product regimen.
Treatment of essential hypertension.
Treatment of renal disease in patients with hypertension and type-2 diabetes mellitus as part of an antihypertensive medicinal-product regimen.
Sabervel is indicated in adults for the treatment of essential hypertension.
It is also indicated for the treatment of renal disease in adult patients with hypertension and type 2 diabetes mellitus as part of an antihypertensive medicinal product regimen.
Treatment of essential hypertension.
Treatment of renal disease in patients with hypertension and type 2 diabetes mellitus as part of an antihypertensive medicinal product regimen (see section 5. 1).
Irbesartan is an angiotensin receptor blocker used to treat hypertension and diabetic nephropathy. It has a long duration of action as it is usually taken once daily and a wide therapeutic index as doses may be as low as 150mg daily but doses of 900mg/day were well tolerated in healthy human subjects.
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Angiotensin II Type 1 Receptor Blockers
Agents that antagonize ANGIOTENSIN II TYPE 1 RECEPTOR. Included are ANGIOTENSIN II analogs such as SARALASIN and biphenylimidazoles such as LOSARTAN. Some are used as ANTIHYPERTENSIVE AGENTS. (See all compounds classified as Angiotensin II Type 1 Receptor Blockers.)
C09CA04
C09CA04
C09CA04
C09CA04
C09CA04
C09CA04
C09CA04
C09CA04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C09 - Agents acting on the renin-angiotensin system
C09C - Angiotensin ii receptor blockers (arbs), plain
C09CA - Angiotensin ii receptor blockers (arbs), plain
C09CA04 - Irbesartan
Absorption
Irbesartan is 60-80% bioavailable with a Tmax of 1.5-2hours. Taking irbesartan with food does not affect the bioavailability. In one study, healthy subjects were given single or multiple oral doses of 150mg, 300mg, 600mg, and 900mg of irbesartan. A single 150mg dose resulted in an AUC of 9.73.0g\hr/mL, a Tmax of 1.5 hours, a half life of 167 hours, and a Cmax of 1.90.4g/mL. A single 300mg dose resulted in an AUC of 20.05.2g\hr/mL, a Tmax of 1.5 hours, a half life of 147 hours, and a Cmax of 2.90.9g/mL. A single 600mg dose resulted in an AUC of 32.611.9g\hr/mL, a Tmax of 1.5 hours, a half life of 148 hours, and a Cmax of 4.91.2g/mL. A single 900mg dose resulted in an AUC of 44.820.0g\hr/mL, a Tmax of 1.5 hours, a half life of 177 hours, and a Cmax of 5.31.9g/mL. Multiple 150mg doses resulted in an AUC of 9.33.0g\hr/mL, a Tmax of 1.5 hours, a half life of 114 hours, and a Cmax of 2.040.4g/mL. Multiple 300mg doses resulted in an AUC of 19.85.8g\hr/mL, a Tmax of 2.0 hours, a half life of 115 hours, and a Cmax of 3.30.8g/mL. Multiple 600mg doses resulted in an AUC of 31.99.7g\hr/mL, a Tmax of 1.5 hours, a half life of 157 hours, and a Cmax of 4.40.7g/mL. Multiple 900mg doses resulted in an AUC of 34.29.3g\hr/mL, a Tmax of 1.8 hours, a half life of 146 hours, and a Cmax of 5.62.1g/mL.
Route of Elimination
20% of a radiolabelled oral dose of irbesartan is recovered in urine, and the rest is recovered in the feces. <2% of the dose is recovered in urine as the unchanged drug.
Volume of Distribution
The volume of distribution of irbesartan is 53-93L.
Clearance
Total plasma clearance of irbesartan is 157-176mL/min while renal clearance is 3.0-3.5mL/min.
Irbesartan is an orally active agent that does not require biotransformation into an active form. The oral absorption of irbesartan is rapid and complete with an average absolute bioavailability of 60% to 80%. Following oral administration of Avapro, peak plasma concentrations of irbesartan are attained at 1.5 to 2 hours after dosing. Food does not affect the bioavailability of Avapro. Irbesartan exhibits linear pharmacokinetics over the therapeutic dose range. The terminal elimination half-life of irbesartan averaged 11 to 15 hours. Steady-state concentrations are achieved within 3 days. Limited accumulation of irbesartan (<20%) is observed in plasma upon repeated once-daily dosing.
NIH; DailyMed. Current Medication Information for Avapro (Irbesartan) Tablet (Revised: September 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b7f1a9ef-c7bb-465c-e4d7-a41395205cad
Studies in animals indicate that radiolabeled irbesartan weakly crosses the blood-brain barrier and placenta.
NIH; DailyMed. Current Medication Information for Avapro (Irbesartan) Tablet (Revised: September 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b7f1a9ef-c7bb-465c-e4d7-a41395205cad
Irbesartan is 90% bound to serum proteins (primarily albumin and a1-acid glycoprotein) with negligible binding to cellular components of blood. The average volume of distribution is 53 liters to 93 liters. Total plasma and renal clearances are in the range of 157 mL/min to 176 mL/min and 3.0 mL/min to 3.5 mL/min, respectively. With repetitive dosing, irbesartan accumulates to no clinically relevant extent.
NIH; DailyMed. Current Medication Information for Avapro (Irbesartan) Tablet (Revised: September 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b7f1a9ef-c7bb-465c-e4d7-a41395205cad
It is not known whether irbesartan is excreted in human milk, but irbesartan or some metabolite of irbesartan is secreted at low concentration in the milk of lactating rats.
NIH; DailyMed. Current Medication Information for Avapro (Irbesartan) Tablet (Revised: September 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b7f1a9ef-c7bb-465c-e4d7-a41395205cad
Irbesaran is largely metabolized by glucuronidation and oxidation in the liver. The majority of metabolism occurs through the action of CYP2C9 with a negligible contribution from CYP3A4. Some hydroxylation also occurs in irbesartan metabolism. Irbesartan can be glucuronidated by UGT1A3 to the M8 metabolite, oxidized to the M3 metabolite, or hydroxylated by CYP2C9 to one of the M4, M5, or M7 metabolites. The M4, M5, and M7 metabolites are all hydroxylated to become the M1 metabolite, which is then oxidized to the M2 metabolite. The M4 metabolite can also be oxidized to the M6 metabolite before hydroxylation to the M2 metabolite. Finally, the minor metabolite SR 49498 is generated from irbesartan by an unknown mechanism.
Irbesartan is metabolized via glucuronide conjugation and oxidation. Following oral or intravenous administration of (14)C-labeled irbesartan, more than 80% of the circulating plasma radioactivity is attributable to unchanged irbesartan. The primary circulating metabolite is the inactive irbesartan glucuronide conjugate (approximately 6%). The remaining oxidative metabolites do not add appreciably to irbesartan's pharmacologic activity. Irbesartan and its metabolites are excreted by both biliary and renal routes. Following either oral or intravenous administration of (14)C-labeled irbesartan, about 20% of radioactivity is recovered in the urine and the remainder in the feces, as irbesartan or irbesartan glucuronide. In vitro studies of irbesartan oxidation by cytochrome P450 isoenzymes indicated irbesartan was oxidized primarily by 2C9; metabolism by 3A4 was negligible. Irbesartan was neither metabolized by, nor did it substantially induce or inhibit, isoenzymes commonly associated with drug metabolism (1A1, 1A2, 2A6, 2B6, 2D6, 2E1). There was no induction or inhibition of 3A4.
NIH; DailyMed. Current Medication Information for Avapro (Irbesartan) Tablet (Revised: September 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b7f1a9ef-c7bb-465c-e4d7-a41395205cad
Irbesartan has known human metabolites that include (1S,4S,5S,6R)-3-[5-[2-[4-[(2-butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl]phenyl]phenyl]-5H-tetrazol-2-ium-2-yl]-2,4,5,6-tetrahydroxycyclohexane-1-carboxylic acid, 2-(3-hydroxybutyl)-3-({4-[2-(2H-1,2,3,4-tetrazol-5-yl)phenyl]phenyl}methyl)-1,3-diazaspiro[4.4]non-1-en-4-one, M3, and M7.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The terminal elimination half life of irbesartan is 11-15 hours.
The terminal elimination half-life of irbesartan averaged 11 to 15 hours.
NIH; DailyMed. Current Medication Information for Avapro (Irbesartan) Tablet (Revised: September 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b7f1a9ef-c7bb-465c-e4d7-a41395205cad
Irbesartan prevents angiotensin II binding to the AT1 receptor in tissues like vascular smooth muscle and the adrenal gland. Irbesartan and its active metabolite bind the AT1 receptor with 8500 times more affinity than they bind to the AT2 receptor. Irbesartan's prevention of angiotensin II binding causes vascular smooth muscle relaxation and prevents the secretion of aldosterone, lowering blood pressure. Angiotensin II would otherwise bind to the AT1 receptor, inducing vasoconstriction and aldosterone secretion, raising blood pressure.
Angiotensin II is a potent vasoconstrictor formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system (RAS) and also stimulates aldosterone synthesis and secretion by adrenal cortex, cardiac contraction, renal resorption of sodium, activity of the sympathetic nervous system, and smooth muscle cell growth. Irbesartan blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively binding to the AT1 angiotensin II receptor. There is also an AT2 receptor in many tissues, but it is not involved in cardiovascular homeostasis. Irbesartan is a specific competitive antagonist of AT1 receptors with a much greater affinity (more than 8500-fold) for the AT1 receptor than for the AT2 receptor and no agonist activity. Blockade of the AT1 receptor removes the negative feedback of angiotensin II on renin secretion, but the resulting increased plasma renin activity and circulating angiotensin II do not overcome the effects of irbesartan on blood pressure. Irbesartan does not inhibit ACE or renin or affect other hormone receptors or ion channels known to be involved in the cardiovascular regulation of blood pressure and sodium homeostasis. Because irbesartan does not inhibit ACE, it does not affect the response to bradykinin; whether this has clinical relevance is not known.
NIH; DailyMed. Current Medication Information for Avapro (Irbesartan) Tablet (Revised: September 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=b7f1a9ef-c7bb-465c-e4d7-a41395205cad