


1. 118499-70-0
2. Ipidacrine Hcl Hydrate
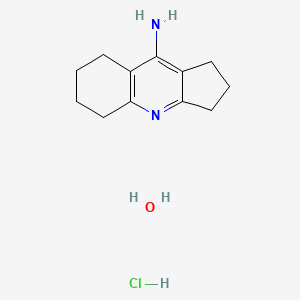
3. 2,3,5,6,7,8-hexahydro-1h-cyclopenta[b]quinolin-9-amine Hydrochloride Hydrate
4. Senita
5. Ipidacrine Hydrochloride Monohydrate
6. Nik-247
7. Ipidacrine Hydrochloride Hydrate [jan]
8. Ipidacrine Monohydrochloride Monohydrate
9. Ov96aik79q
10. Ncgc00167585-01
11. 2,3,5,6,7,8-hexahydro-1h-cyclopenta[b]quinolin-9-amine;hydrate;hydrochloride
12. 1h-cyclopenta(b)quinolin-9-amine, 2,3,5,6,7,8-hexahydro-, Hydrochloride, Hydrate (1:1:1)
13. 1h-cyclopenta(b)quinolin-9-amine, 2,3,5,6,7,8-hexahydro-, Monohydrochloride, Monohydrate
14. Dsstox_cid_26734
15. Dsstox_rid_81863
16. Dsstox_gsid_46734
17. Ipidacrine Hydrochloride Hydrate (jan)
18. Cas-118499-70-0
19. Unii-ov96aik79q
20. Schembl6633743
21. Chembl1898637
22. Dtxsid9046734
23. Tox21_112577
24. Mfcd00517543
25. Akos015911890
26. Tox21_112577_1
27. Ac-7050
28. 2,3,5,6,7,8-hexahydro-1h-cyclopenta[b]quinolin-9-amine Hydrochloride Hydrate (1:1:1)
29. Ncgc00263455-01
30. As-38638
31. D09750
32. Ipidacrine Hydrochloride Monohydrate [mi]
33. 499i700
34. A893144
35. Q27285863
36. F6170-0023
37. 2,3,5,6,7,8-hexahydro-1h-cyclopenta[b]quinolin-9-aminehydrochloridehydrate
38. 2,3,5,6,7,8-hexahydro-1h-cyclopenta(b)quinolin-9-ylamine, Hydrochloride Hydrate
39. 2,3,5,6,7,8-hexahydro-1h-cyclopenta[b]quinolin-9-amine Hydrochloride Hydrate (1
40. 9-amino-2,3,5,6,7,8-hexahydro-1h-cyclopenta[b]quinoline Hydrochloride Monohydrate; 2,3,5,6,7,8-hexahydro-1h-cyclopenta[b]quinolin-9-amine Hydrochloride Hydrate (1:1:1)
| Molecular Weight | 242.74 g/mol |
|---|---|
| Molecular Formula | C12H19ClN2O |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 242.1185909 g/mol |
| Monoisotopic Mass | 242.1185909 g/mol |
| Topological Polar Surface Area | 39.9 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 216 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |