
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
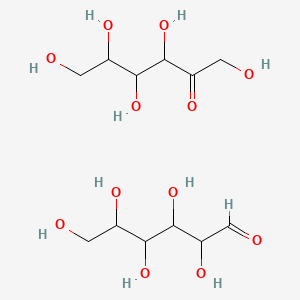

1. Invert Sugar
1. 8013-17-0
2. Invert Sugar
3. Hs 500
4. 2,3,4,5,6-pentahydroxyhexanal;1,3,4,5,6-pentahydroxyhexan-2-one
5. Sugar,invert
6. Schembl20361633
7. Mfcd00148911
8. 1,3,4,5,6-pentahydroxyhexan-2-one; 2,3,4,5,6-pentahydroxyhexanal
9. 2,3,4,5,6-pentahydroxyhexanal Compound With 1,3,4,5,6-pentahydroxyhexan-2-one (1:1)
| Molecular Weight | 360.31 g/mol |
|---|---|
| Molecular Formula | C12H24O12 |
| Hydrogen Bond Donor Count | 10 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 10 |
| Exact Mass | 360.12677620 g/mol |
| Monoisotopic Mass | 360.12677620 g/mol |
| Topological Polar Surface Area | 236 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 285 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 7 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Cariogenic Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
SOLN CONTAINING 25% TO 50% DEXTROSE ARE USED IN PARENTERAL HYPERALIMENTATION. THESE MAY BE ADMIN SLOWLY TO PROVIDE AS MUCH AS 3,000-4,000 CALORIES DAILY, & MUST BE GIVEN VIA THE SUPERIOR VENA CAVA OR OTHER EQUALLY LARGE VEIN. /DEXTROSE/
American Medical Association, Council on Drugs. AMA Drug Evaluations. 2nd ed. Acton, Mass.: Publishing Sciences Group, Inc., 1973., p. 181
SOLN CONTAINING 50% TO 75% DEXTROSE DECR THE FREQUENCY OF DIALYSIS IN PATIENTS WITH RENAL FAILURE. /DEXTROSE/
American Medical Association, Council on Drugs. AMA Drug Evaluations. 2nd ed. Acton, Mass.: Publishing Sciences Group, Inc., 1973., p. 181
IF THE INDIVIDUAL PATIENT'S CAPACITY TO UTILIZE DEXTROSE IS EXCEEDED, GLYCOSURIA & DIURESIS WILL OCCUR. /DEXTROSE/
American Medical Association, Council on Drugs. AMA Drug Evaluations. 2nd ed. Acton, Mass.: Publishing Sciences Group, Inc., 1973., p. 181
10% SOLN OF INVERT SUGAR BUFFERED TO PH 6.8 WAS FOUND TO CAUSE SIGNIFICANTLY LOWER INCIDENCE OF THROMBOPHLEBITIS THAN SOLN OF SAME STRENGTH WITH PH BETWEEN 3.5 AND 5.4 FOLLOWING INTRAVENOUS INJECTION OF 500 ML QUANTITIES IN 76 PATIENTS.
Blacow, N. W. (ed.). Martindale: The Extra Pharmacopoeia. 26th ed. London: The Pharmaceutical Press, 1972., p. 81
DEXTROSE INJECTION SHOULD NOT BE USED AS A DILUENT FOR BLOOD BECAUSE IT CAUSES CLUMPING OF RED BLOOD CELLS &, POSSIBLY, HEMOLYSIS. THIS CAN BE AVOIDED BY USING 5% DEXTROSE IN 0.2% OR 0.11% SODIUM CHLORIDE INJECTION. /DEXTROSE/
American Medical Association, Council on Drugs. AMA Drug Evaluations. 2nd ed. Acton, Mass.: Publishing Sciences Group, Inc., 1973., p. 181
SUBCUTANEOUS /ADMIN OF INVERT SUGAR/: GENERALLY DEEMED INADVISABLE.
American Medical Association, Council on Drugs. AMA Drug Evaluations. 2nd ed. Acton, Mass.: Publishing Sciences Group, Inc., 1973., p. 181
For more Drug Warnings (Complete) data for INVERT SUGAR (7 total), please visit the HSDB record page.
Invert sugar presents a large variety of uses. It can be used therapeutically for parenteral hyperalimentation or to be used as an excipient with a known effect. Invert sugar is also approved for its use in food products as a humectant, crystallization modifier, and liquid and nutritive sweetener.
Intravenous administration of invert sugar has been proven to be favorable. These reports indicate a temporary rise, within physiological levels, of lactate, pyruvate, and insulin in the blood plasma whereas the level of free fatty acids was declined.
Cariogenic Agents
Substances that promote DENTAL CARIES. (See all compounds classified as Cariogenic Agents.)
Sweetening Agents
Substances that sweeten food, beverages, medications, etc., such as sugar, saccharine or other low-calorie synthetic products. (From Random House Unabridged Dictionary, 2d ed) (See all compounds classified as Sweetening Agents.)
C - Cardiovascular system
C05 - Vasoprotectives
C05B - Antivaricose therapy
C05BB - Sclerosing agents for local injection
C05BB03 - Invert sugar
Absorption
It has been reported that administration of 10-15% of intravenous invert sugar solution is rapidly absorbed in the intestine and distributed in blood without exceeding the renal threshold. The absorption of invert sugar happens mainly as monosaccharides. Glucose is absorbed into the portal vein by the transporter GLUT2 while fructose is absorbed by the transporter GLUT5.
Route of Elimination
Reports have indicated that after intravenous administration of invert sugar present a reduced content or carbohydrates in urine when compared to the intravenous administration of glucose. This response indicates a reduced diuresis of invert sugar. It has also been reported a reduced diuresis in the presence of invert diuresis.
LESS THAN 2% OF SUGAR IS EXCRETED IN URINE WHEN 1 L OF 10% INVERT SUGAR SOLN IS INFUSED IN 1 HR. WHEN GIVEN OVER A LONGER PERIOD OF TIME INVERT SUGAR IS COMPLETELY UTILIZED AND NONE IS EXCRETED IN URINE.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 40:20
The metabolism of invert sugar and other fructose-glucose sweeteners is not meaningfully different. Once absorbed, the fructose monosaccharides are taken up by the liver and bypass a key step regulatory step in glycolysis. Both of the monosaccharides first step metabolism is marked by its phosphorylation to glucose-6-phosphate by glucokinase or fructose-1-phosphate by fructokinase. After this step, the glycolysis continues its pathway until the obtention of pyruvic acid. The metabolism of fructose is more rapid than the one of glucose and thus, it is possible to administer at the same speed than a glucose dose correspondent to 55% of the invert sugar dose.
The glucose half-life following intravenous administration of invert sugar is of approximately 30 min. In the same report, the half-life of fructose after the intravenous administration is reported to be 16 min.
ABOUT THIS PAGE
34
PharmaCompass offers a list of Invert Sugar API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Invert Sugar manufacturer or Invert Sugar supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Invert Sugar manufacturer or Invert Sugar supplier.
PharmaCompass also assists you with knowing the Invert Sugar API Price utilized in the formulation of products. Invert Sugar API Price is not always fixed or binding as the Invert Sugar Price is obtained through a variety of data sources. The Invert Sugar Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Invert Sugar manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Invert Sugar, including repackagers and relabelers. The FDA regulates Invert Sugar manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Invert Sugar API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Invert Sugar supplier is an individual or a company that provides Invert Sugar active pharmaceutical ingredient (API) or Invert Sugar finished formulations upon request. The Invert Sugar suppliers may include Invert Sugar API manufacturers, exporters, distributors and traders.
Invert Sugar Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Invert Sugar GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Invert Sugar GMP manufacturer or Invert Sugar GMP API supplier for your needs.
A Invert Sugar CoA (Certificate of Analysis) is a formal document that attests to Invert Sugar's compliance with Invert Sugar specifications and serves as a tool for batch-level quality control.
Invert Sugar CoA mostly includes findings from lab analyses of a specific batch. For each Invert Sugar CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Invert Sugar may be tested according to a variety of international standards, such as European Pharmacopoeia (Invert Sugar EP), Invert Sugar JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Invert Sugar USP).
