
Synopsis
Synopsis
0
JDMF
0
KDMF
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Fungicidin
2. Mycostatin
3. Nilstat
4. Nystatin
5. Nystatin A2
6. Nystatin A3
7. Nystatin G
8. Stamicin
9. Stamycin
1. Nystatin
2. 34786-70-4
3. W1lx4t91wi
4. Nystatinum
5. Terrastatin
6. Nyotran
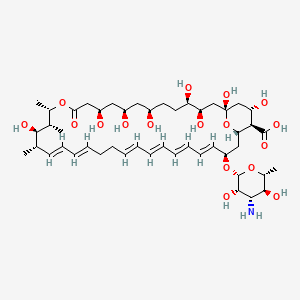
7. (1s,3r,4r,7r,9r,11r,15s,16r,17r,18s,19e,21e,25e,27e,29e,31e,33r,35s,36r,37s)-33-[(2r,3s,4s,5s,6r)-4-amino-3,5-dihydroxy-6-methyloxan-2-yl]oxy-1,3,4,7,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,39-dioxabicyclo[33.3.1]nonatriaconta-19,21,25,27,29,31-hexaene-36-carboxylic Acid
8. Mikostatin
9. L-nystatin
10. Unii-w1lx4t91wi
11. Nystatin A1 [mi]
12. Chembl450895
13. Schembl18633553
14. Dtxsid80872323
15. Chebi:473992
16. Lmpk06000004
17. Akos032963569
18. Zinc253387941
19. Q27292191
20. (7r,10r)-8,9-dideoxy-28,29-dihydro-7,10-dihydroxyamphotericin B
21. (1s,3r,4e,6e,8e,10e,14e,16e,18s,19r,20r,21s,25r,27r,29r,32r,33r,35s,37s,38r)-3-[(2r,3s,4s,5s,6r)-4-amino-3,5-dihydroxy-6-methyl-tetrahydropyran-2-yl]oxy-19,25,27,29,32,33,35,37-octahydroxy-18,20,21-trimethyl-23-oxo-22,39-dioxabicyclo[33.3.1]nonatriaconta-4,6,8,10,14,16-hexaene-38-carboxylic Acid
22. (1s,3r,4r,7r,9r,11r,15s,16r,17r,18s,19e,21e,25e,27e,29e,31e,33r,35s,36r,37s)-33-[(3-amino-3,6-dideoxy-beta-d-mannopyranosyl)oxy]-1,3,4,7,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,39-dioxabicyclo[33.3.1]nonatriaconta-19,21,25,27,29,31-hexaene-36-carboxylic Acid
23. (1s,3r,4r,7r,9r,11r,15s,16r,17r,18s,19e,21z,25e,27e,29e,31e,33r,35s,36r,37s)-33-[(2r,3s,4s,5s,6r)-4-amino-3,5-dihydroxy-6-methyloxan-2-yl]oxy-1,3,4,7,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,39-dioxabicyclo[33.3.1]nonatriaconta-19,21,25,27,29,31-hexaene-36-carboxylic Acid
| Molecular Weight | 926.1 g/mol |
|---|---|
| Molecular Formula | C47H75NO17 |
| XLogP3 | -0.2 |
| Hydrogen Bond Donor Count | 12 |
| Hydrogen Bond Acceptor Count | 18 |
| Rotatable Bond Count | 3 |
| Exact Mass | 925.50349992 g/mol |
| Monoisotopic Mass | 925.50349992 g/mol |
| Topological Polar Surface Area | 320 Ų |
| Heavy Atom Count | 65 |
| Formal Charge | 0 |
| Complexity | 1620 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 19 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 6 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antibiotics, Antifungal; Antibiotics, Macrolide; Ionophores
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
MEDICATION (VET):Antifungal; growth promotant
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1208
MEDICATION (VET): /Used in treatment of/ intestinal mycosis due to Candida albicans in poultry; occasionally orally in cats and dogs in suspected Candida intestinal overgrowth following antibiotic therapy, and also topically ... as cream or ointment on skin lesions ...
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 397
Nystatin vaginal tablets are used as lozenges to treat oropharyngeal candidiasis since their slow dissolution rate provides prolonged oral contact. /NOT included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2226
For more Therapeutic Uses (Complete) data for NYSTATIN (9 total), please visit the HSDB record page.
Since it is not known whether nystatin is distributed into human milk, the drug should be used with caution in nursing women.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 543
Adverse effects occur infrequently with oral nystatin therapy. Mild and transitory nausea, vomiting, GI distress, and diarrhea have occurred; high oral doses (e.g., greater than 5 million units daily) are most likely to produce these adverse GI effects. Hypersensitivity reactions have been reported very rarely.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 543
Patients should be instructed to contact their physician if symptoms of irritation or sensitization occur during nystatin therapy. Patients should be warned against interrupting or discontinuing vaginal nystatin therapy during a prescribed regimen, even during menstruation or if symptomatic relief occurs after only a few days of therapy, unless otherwise instructed by their physician. Patients should be advised that adjunctive measures such as therapeutic douches are not necessary and may be inadvisable during vaginal nystatin therapy; however, cleansing douches may be used in nonpregnant women, if desired, for aesthetic effect.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 3432
Adverse reactions to topically applied nystatin are very infrequent, even during prolonged use. Irritation has occurred rarely. Hypersensitivity reactions to nystatin have been reported only rarely; however, preservatives (eg, ethylenediamine, parabens, thimerosal) in some of the formulations are associated with a high incidence of contact dermatitis. An acneiform eruption has occurred rarely following topical application of nystatin and triamcinolone acetonide.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 3432
For more Drug Warnings (Complete) data for NYSTATIN (9 total), please visit the HSDB record page.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
Ionophores
Chemical agents that increase the permeability of biological or artificial lipid membranes to specific ions. Most ionophores are relatively small organic molecules that act as mobile carriers within membranes or coalesce to form ion permeable channels across membranes. Many are antibiotics, and many act as uncoupling agents by short-circuiting the proton gradient across mitochondrial membranes. (See all compounds classified as Ionophores.)
A - Alimentary tract and metabolism
A07 - Antidiarrheals, intestinal antiinflammatory/antiinfective agents
A07A - Intestinal antiinfectives
A07AA - Antibiotics
A07AA02 - Nystatin
D - Dermatologicals
D01 - Antifungals for dermatological use
D01A - Antifungals for topical use
D01AA - Antibiotics
D01AA01 - Nystatin
G - Genito urinary system and sex hormones
G01 - Gynecological antiinfectives and antiseptics
G01A - Antiinfectives and antiseptics, excl. combinations with corticosteroids
G01AA - Antibiotics
G01AA01 - Nystatin
Nystatin penetrates eye poorly.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 969
Nystatin is poorly absorbed from the GI tract, and detectable blood concentrations are not obtained after usual doses. Following oral administration, nystatin is excreted almost entirely in feces as unchanged drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 543
In healthy individuals, mean salivary nystatin concentrations in excess of those required in vitro for growth inhibition of clinically important Candida persist for approximately 2 hours after the beginning of oral dissolution of two nystatin lozenges (400,000 units) administered simultaneously.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 3432
Not absorbed following topical application to intact skin or mucous membranes.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2225
For more Absorption, Distribution and Excretion (Complete) data for NYSTATIN (6 total), please visit the HSDB record page.
Nystatin exerts its antifungal activity by binding to sterols in the fungal cell membrane. The drug is not active against organisms (e.g., bacteria) that do not contain sterols in their cell membrane. As a result of this binding, the membrane is no longer able to function as a selective barrier, and potassium and other cellular constituents are lost.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 543
... /Antimicrobial/ agents that act directly on the cell membrane of the microorganism, affecting permeability and leading to leakage of intracellular compounds; these include ... the polyene antifungal agents nystatin ... which bind to cell-wall sterols ...
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1143

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
98
PharmaCompass offers a list of Nystatin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Nystatin manufacturer or Nystatin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Nystatin manufacturer or Nystatin supplier.
PharmaCompass also assists you with knowing the Nystatin API Price utilized in the formulation of products. Nystatin API Price is not always fixed or binding as the Nystatin Price is obtained through a variety of data sources. The Nystatin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Fungicidin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Fungicidin, including repackagers and relabelers. The FDA regulates Fungicidin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Fungicidin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Fungicidin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Fungicidin supplier is an individual or a company that provides Fungicidin active pharmaceutical ingredient (API) or Fungicidin finished formulations upon request. The Fungicidin suppliers may include Fungicidin API manufacturers, exporters, distributors and traders.
click here to find a list of Fungicidin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Fungicidin DMF (Drug Master File) is a document detailing the whole manufacturing process of Fungicidin active pharmaceutical ingredient (API) in detail. Different forms of Fungicidin DMFs exist exist since differing nations have different regulations, such as Fungicidin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Fungicidin DMF submitted to regulatory agencies in the US is known as a USDMF. Fungicidin USDMF includes data on Fungicidin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Fungicidin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Fungicidin suppliers with USDMF on PharmaCompass.
A Fungicidin CEP of the European Pharmacopoeia monograph is often referred to as a Fungicidin Certificate of Suitability (COS). The purpose of a Fungicidin CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Fungicidin EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Fungicidin to their clients by showing that a Fungicidin CEP has been issued for it. The manufacturer submits a Fungicidin CEP (COS) as part of the market authorization procedure, and it takes on the role of a Fungicidin CEP holder for the record. Additionally, the data presented in the Fungicidin CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Fungicidin DMF.
A Fungicidin CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Fungicidin CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Fungicidin suppliers with CEP (COS) on PharmaCompass.
A Fungicidin written confirmation (Fungicidin WC) is an official document issued by a regulatory agency to a Fungicidin manufacturer, verifying that the manufacturing facility of a Fungicidin active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Fungicidin APIs or Fungicidin finished pharmaceutical products to another nation, regulatory agencies frequently require a Fungicidin WC (written confirmation) as part of the regulatory process.
click here to find a list of Fungicidin suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Fungicidin as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Fungicidin API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Fungicidin as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Fungicidin and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Fungicidin NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Fungicidin suppliers with NDC on PharmaCompass.
Fungicidin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Fungicidin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Fungicidin GMP manufacturer or Fungicidin GMP API supplier for your needs.
A Fungicidin CoA (Certificate of Analysis) is a formal document that attests to Fungicidin's compliance with Fungicidin specifications and serves as a tool for batch-level quality control.
Fungicidin CoA mostly includes findings from lab analyses of a specific batch. For each Fungicidin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Fungicidin may be tested according to a variety of international standards, such as European Pharmacopoeia (Fungicidin EP), Fungicidin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Fungicidin USP).

