
Synopsis
Synopsis
0
EU WC
0
KDMF
0
VMF
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
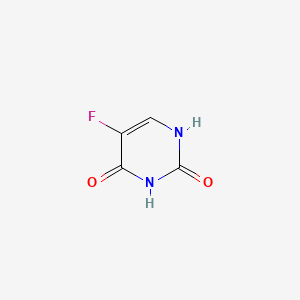

1. 5 Fluorouracil
2. 5 Fluorouracil Biosyn
3. 5 Fu Lederle
4. 5 Fu Medac
5. 5 Hu Hexal
6. 5-fluorouracil
7. 5-fluorouracil-biosyn
8. 5-fu
9. 5-fu Lederle
10. 5-fu Medac
11. 5-hu Hexal
12. 5fu
13. Adrucil
14. Carac
15. Dakota, Fluorouracile
16. Efudex
17. Efudix
18. Fluoro Uracile Icn
19. Fluoro-uracile Icn
20. Fluoroplex
21. Fluorouracil Gry
22. Fluorouracil Mononitrate
23. Fluorouracil Monopotassium Salt
24. Fluorouracil Monosodium Salt
25. Fluorouracil Potassium Salt
26. Fluorouracil-gry
27. Fluorouracile Dakota
28. Fluorouracilo Ferrer Far
29. Fluoruracil
30. Fluracedyl
31. Flurodex
32. Haemato Fu
33. Haemato-fu
34. Neofluor
35. Onkofluor
36. Ribofluor
1. 5-fluorouracil
2. 51-21-8
3. 5-fu
4. Fluoroplex
5. Efudex
6. Adrucil
7. Carac
8. 5-fluoropyrimidine-2,4(1h,3h)-dione
9. Fluracil
10. Fluoroblastin
11. Queroplex
12. Kecimeton
13. Phthoruracil
14. Carzonal
15. Timazin
16. Arumel
17. Efudix
18. Fluril
19. 5-fluoracil
20. Fluracilum
21. Ulup
22. 5-fluoro-1h-pyrimidine-2,4-dione
23. Fluorouracilum
24. Fluro Uracil
25. 5 Fluorouracil
26. 5-fluoruracil
27. Ftoruracil
28. Efurix
29. Fluri
30. 5-fluoro-2,4(1h,3h)-pyrimidinedione
31. 5-fluoropyrimidine-2,4-dione
32. Effluderm (free Base)
33. Fluorouracilo
34. 2,4(1h,3h)-pyrimidinedione, 5-fluoro-
35. 2,4-dihydroxy-5-fluoropyrimidine
36. Ro 2-9757
37. Uracil, 5-fluoro-
38. 5-fluoropyrimidine-2,4-diol
39. 5-fluor-2,4-pyrimidindiol
40. 5-fluoro Uracil
41. 5-fluoro-uracil
42. 5-ftouracyl
43. 2,4-dioxo-5-fluoropyrimidine
44. Nsc 19893
45. Nsc-19893
46. Fluorouracil, 5-
47. 5-fluoro-2,4-pyrimidinedione
48. Fluorouracil (adrucil)
49. Fluorouricil
50. Ro-2-9757
51. Tolak
52. 5-fluor-2,4-dihydroxypyrimidin
53. U-8953
54. 191047-65-1
55. Fu
56. Chembl185
57. 2,4-pyrimidinediol, 5-fluoro- (9ci)
58. Mls000069498
59. 5 Fu
60. 5fu
61. Fluroblastin
62. Phtoruracil
63. Chebi:46345
64. Fluoro-uracile
65. Fluoro-uracilo
66. U3p01618rt
67. Nsc19893
68. 5-fluoro-1,2,3,4-tetrahydropyrimidine-2,4-dione
69. 5-faracil
70. Cinco Fu
71. Ro-29757
72. 191047-64-0
73. 191115-88-5
74. Urf
75. Ncgc00015442-02
76. Fluorouracile
77. Effluderm
78. Smr000038082
79. Dsstox_cid_634
80. Fluorouracile [dcit]
81. 5-fluoracil [german]
82. 5-fluoracyl
83. 5-fluoruracil [german]
84. Dsstox_rid_75705
85. Dsstox_gsid_20634
86. Fluorouracilum [inn-latin]
87. Fluorouracilo [inn-spanish]
88. 5-fluoropyrimidin-2,4-diol
89. Fluorouracil Cream
90. Cas-51-21-8
91. Fluoroplex (tn)
92. 5-fluor-2,4-pyrimidindiol [czech]
93. Adrucil (tn)
94. Ccris 2582
95. Carac (tn)
96. 5-fluor-2,4-dihydroxypyrimidin [czech]
97. Hsdb 3228
98. Sr-01000075881
99. 5-fluor-2,4(1h,3h)-pyrimidindion [czech]
100. Einecs 200-085-6
101. 5-fluor-2,4(1h,3h)-pyrimidindion
102. Fluouracil
103. Inhibits Thymilidate Synthetase
104. Unii-u3p01618rt
105. 2,4-dioxo-5-fluoropryimidine
106. 5-fluorourasil
107. Ai3-25297
108. Fluoro Uracil
109. 5-florouracil
110. 5-fluorouacil
111. 5-fu (tn)
112. 5-fluracil
113. 1upf
114. 5f-uracil
115. U 8953
116. 1-fluoro-1h-pyrimidine-2,4-dione
117. Adrucil (icn)
118. Mfcd00006018
119. Adrucil (fluorouracil)
120. Fluorouracil - Adrucil
121. Fluorouracil [usan:usp:inn:ban:jan]
122. Spectrum_000841
123. Opera_id_134
124. 5-fluorouracil, 99%
125. Spectrum2_000076
126. Spectrum3_000434
127. Spectrum4_000557
128. Spectrum5_000718
129. Wln: T6mvmvj Ef
130. Fluorouracil [mi]
131. Lopac-f-6627
132. F0151
133. Fluorouracil [inn]
134. Fluorouracil [jan]
135. Upcmld-dp130
136. Ec 200-085-6
137. F 6627
138. Fluorouracil [hsdb]
139. Fluorouracil [usan]
140. Schembl3646
141. 5-fluorpyrimidin-2,4-diol
142. Fluorouracil [vandf]
143. Lopac0_000536
144. Bspbio_002048
145. Fluorouracil [mart.]
146. Kbiogr_001253
147. Kbioss_001321
148. 2(1h)-pyrimidinone, 5-fluoro-4-hydroxy- (9ci)
149. 4(3h)-pyrimidinone, 5-fluoro-2-hydroxy- (9ci)
150. Mls002415705
151. Divk1c_000054
152. Fluorouracil [usp-rs]
153. Fluorouracil [who-dd]
154. Fluorouracil [who-ip]
155. Spectrum1500305
156. Spbio_000291
157. 5-fluorouracil [iarc]
158. 5-fluoro-2,4-dioxo-pyrimidin
159. 5-fluoro-pyrimidine-2,4-diol
160. Gtpl4789
161. Dtxsid2020634
162. Upcmld-dp130:001
163. Fluorouracil (jp17/usp/inn)
164. Hms500c16
165. Kbio1_000054
166. Kbio2_001321
167. Kbio2_003889
168. Kbio2_006457
169. Kbio3_001268
170. 5-fluoro-2,3h)-pyrimidinedione
171. 5-fluorouracil [who-ip]
172. 2,4-pyrimidinedione, 5-fluoro-
173. Ninds_000054
174. Bcpp000428
175. Fluorouracil [orange Book]
176. Hms1920o18
177. Hms2090i04
178. Hms2091f19
179. Hms3259o03
180. Hms3261l13
181. Hms3654k22
182. Hms3715h03
183. Hms3865l03
184. Pharmakon1600-01500305
185. Fluorouracil [ep Monograph]
186. 5-fluorouracil, Analytical Standard
187. Bcp02083
188. 2,3h)-pyrimidinedione, 5-fluoro-
189. Fluorouracil (5-fluoracil, 5-fu)
190. Fluorouracil (5-fluoracil; 5-fu)
191. Fluorouracil [usp Monograph]
192. Tox21_110150
193. Tox21_202335
194. Tox21_300112
195. Tox21_500536
196. Bdbm50340677
197. Ccg-39879
198. Dl-399
199. Nsc757036
200. Nsc816997
201. S1209
202. Stk297802
203. Stl367375
204. Zinc38212689
205. Fluorouracilum [who-ip Latin]
206. Akos000119162
207. Akos003237897
208. Akos008044307
209. Tox21_110150_1
210. Bcp9000239
211. Cs-0993
212. Db00544
213. Ks-5129
214. Lp00536
215. Nc00454
216. Nsc-757036
217. Nsc-816997
218. Sdccgsbi-0050519.p005
219. Flucytosine Impurity A [who-ip]
220. Idi1_000054
221. Ncgc00015442-01
222. Ncgc00015442-03
223. Ncgc00015442-04
224. Ncgc00015442-05
225. Ncgc00015442-06
226. Ncgc00015442-07
227. Ncgc00015442-08
228. Ncgc00015442-09
229. Ncgc00015442-10
230. Ncgc00015442-11
231. Ncgc00015442-12
232. Ncgc00015442-15
233. Ncgc00015442-16
234. Ncgc00015442-24
235. Ncgc00015442-30
236. Ncgc00091349-01
237. Ncgc00091349-02
238. Ncgc00091349-03
239. Ncgc00091349-04
240. Ncgc00091349-05
241. Ncgc00091349-07
242. Ncgc00091349-08
243. Ncgc00254023-01
244. Ncgc00259884-01
245. Ncgc00261221-01
246. 1004-03-1
247. 5-fluoro-2,4-(1h,3h)-pyrimidinedione
248. Ac-11201
249. Bf166014
250. Emtricitabine Impurity F [who-ip]
251. Hy-90006
252. Nci60_001652
253. Sri-10792-04
254. Sri-10792-05
255. Sri-10792-06
256. Sri-10792_07
257. Sri-10792_08
258. 5-fluoro-1h-pyrimidine-2,4-dione(5fu)
259. 5-fluorouracil, >=99% (hplc), Powder
260. Sbi-0050519.p004
261. 5-fluoro-1h-pyrimidine-2,4-dione(5-fu)
262. Db-051923
263. Db-065735
264. 5-fluoro-1h-pyrimidine-2,4-dione (5-fu)
265. Am20100252
266. Eu-0100536
267. Flucytosine Impurity A [ep Impurity]
268. Ft-0601511
269. Ft-0668745
270. Ft-0695666
271. Ft-0695667
272. Ft-0707652
273. Sw199617-3
274. 5-fluoro-1h-pyrimidine-2,4-dione(5-fura)
275. Fluorouracil, Meets Usp Testing Specifications
276. 51f218
277. C07649
278. D00584
279. 5-fluorouracil, Vetec(tm) Reagent Grade, >=99%
280. Q238512
281. W-60379
282. (5-fluorouracil)5-fluoro-1h-pyrimidine-2,4-dione
283. 5-fluoro-1h-pyrimidine-2,4-dione(5-fluoro Uracil)
284. Sr-01000075881-1
285. Sr-01000075881-3
286. Sr-01000075881-5
287. W-202929
288. 5-fluoro-1h-pyrimidine-2,4-dione (5-fluorouracil)
289. Brd-k24844714-001-02-1
290. 5-fluoropyrimidin-2,4(1h,3h)-dione [who-ip]
291. Z275128052
292. 5-fluoro-1h-pyrimidine-2,4-dione(5-fluorouracil)(5-fu)
293. 5-fluorouracil, Certified Reference Material, Tracecert(r)
294. Fluorouracil, British Pharmacopoeia (bp) Reference Standard
295. Fluorouracil, European Pharmacopoeia (ep) Reference Standard
296. Fluorouracil, United States Pharmacopeia (usp) Reference Standard
297. Pyrimidine Antimetabolite: Inhibits Nucleic Acid Replication; Tetratogen
298. Fluorouracil, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 130.08 g/mol |
|---|---|
| Molecular Formula | C4H3FN2O2 |
| XLogP3 | -0.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 130.01785550 g/mol |
| Monoisotopic Mass | 130.01785550 g/mol |
| Topological Polar Surface Area | 58.2 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 199 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 8 | |
|---|---|
| Drug Name | Carac |
| PubMed Health | Fluorouracil |
| Drug Classes | Antineoplastic Agent, Antineoplastic, Dermatological |
| Drug Label | Carac (fluorouracil cream) Cream, 0.5%, contains fluorouracil for topical dermatologic use. Chemically, fluorouracil is 5-fluoro-2,4(1H, 3H)-pyrimidinedione. The molecular formula is C4H3FN2O2. Fluorouracil has a molecular weight of 130.08.Carac Cr... |
| Active Ingredient | Fluorouracil |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 0.5% |
| Market Status | Prescription |
| Company | Valeant Bermuda |
| 2 of 8 | |
|---|---|
| Drug Name | Efudex |
| PubMed Health | Fluorouracil |
| Drug Classes | Antineoplastic Agent, Antineoplastic, Dermatological |
| Drug Label | Efudex Solutions and Cream are topical preparations containing the fluorinated pyrimidine 5-fluorouracil, an antineoplastic antimetabolite.Efudex Solution consists of 2% or 5% fluorouracil on a weight/weight basis, compounded with propylene glycol, t... |
| Active Ingredient | Fluorouracil |
| Dosage Form | Cream; Solution |
| Route | Topical |
| Strength | 5%; 2% |
| Market Status | Prescription |
| Company | Valeant Pharm Intl |
| 3 of 8 | |
|---|---|
| Drug Name | Fluoroplex |
| PubMed Health | Fluorouracil |
| Drug Classes | Antineoplastic Agent, Antineoplastic, Dermatological |
| Drug Label | FLUOROPLEX (fluorouracil) 1% Topical Cream is an antineoplastic/antimetabolite product for dermatological use. Fluorouracil has the empirical formula C4H3FN2O2 and a molecular weight of 130.08. It is sparingly soluble in water and slightly soluble... |
| Active Ingredient | Fluorouracil |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 1% |
| Market Status | Prescription |
| Company | Aqua Pharms |
| 4 of 8 | |
|---|---|
| Drug Name | Fluorouracil |
| Drug Label | Fluorouracil Injection, USP an antineoplastic antimetabolite, is a sterile, nonpyrogenic injectable solution for intravenous administration. Each mL contains 50 mg of fluorouracil and water for injection. Sodium hydroxide may be added to adjust pH to... |
| Active Ingredient | Fluorouracil |
| Dosage Form | Cream; Injectable; Solution |
| Route | Injection; Topical |
| Strength | 5gm/100ml (50mg/ml); 1gm/20ml (50mg/ml); 500mg/10ml (50mg/ml); 5%; 2%; 2.5gm/50ml (50mg/ml) |
| Market Status | Prescription |
| Company | Accord Hlthcare; Spear Pharms; Teva Pharms Usa; Taro; Sandoz; Fresenius Kabi Usa; Ebewe Pharma; Onco Therapies |
| 5 of 8 | |
|---|---|
| Drug Name | Carac |
| PubMed Health | Fluorouracil |
| Drug Classes | Antineoplastic Agent, Antineoplastic, Dermatological |
| Drug Label | Carac (fluorouracil cream) Cream, 0.5%, contains fluorouracil for topical dermatologic use. Chemically, fluorouracil is 5-fluoro-2,4(1H, 3H)-pyrimidinedione. The molecular formula is C4H3FN2O2. Fluorouracil has a molecular weight of 130.08.Carac Cr... |
| Active Ingredient | Fluorouracil |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 0.5% |
| Market Status | Prescription |
| Company | Valeant Bermuda |
| 6 of 8 | |
|---|---|
| Drug Name | Efudex |
| PubMed Health | Fluorouracil |
| Drug Classes | Antineoplastic Agent, Antineoplastic, Dermatological |
| Drug Label | Efudex Solutions and Cream are topical preparations containing the fluorinated pyrimidine 5-fluorouracil, an antineoplastic antimetabolite.Efudex Solution consists of 2% or 5% fluorouracil on a weight/weight basis, compounded with propylene glycol, t... |
| Active Ingredient | Fluorouracil |
| Dosage Form | Cream; Solution |
| Route | Topical |
| Strength | 5%; 2% |
| Market Status | Prescription |
| Company | Valeant Pharm Intl |
| 7 of 8 | |
|---|---|
| Drug Name | Fluoroplex |
| PubMed Health | Fluorouracil |
| Drug Classes | Antineoplastic Agent, Antineoplastic, Dermatological |
| Drug Label | FLUOROPLEX (fluorouracil) 1% Topical Cream is an antineoplastic/antimetabolite product for dermatological use. Fluorouracil has the empirical formula C4H3FN2O2 and a molecular weight of 130.08. It is sparingly soluble in water and slightly soluble... |
| Active Ingredient | Fluorouracil |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 1% |
| Market Status | Prescription |
| Company | Aqua Pharms |
| 8 of 8 | |
|---|---|
| Drug Name | Fluorouracil |
| Drug Label | Fluorouracil Injection, USP an antineoplastic antimetabolite, is a sterile, nonpyrogenic injectable solution for intravenous administration. Each mL contains 50 mg of fluorouracil and water for injection. Sodium hydroxide may be added to adjust pH to... |
| Active Ingredient | Fluorouracil |
| Dosage Form | Cream; Injectable; Solution |
| Route | Injection; Topical |
| Strength | 5gm/100ml (50mg/ml); 1gm/20ml (50mg/ml); 500mg/10ml (50mg/ml); 5%; 2%; 2.5gm/50ml (50mg/ml) |
| Market Status | Prescription |
| Company | Accord Hlthcare; Spear Pharms; Teva Pharms Usa; Taro; Sandoz; Fresenius Kabi Usa; Ebewe Pharma; Onco Therapies |
Antimetabolites; Antimetabolites, Antineoplastic; Immunosuppressive Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Fluorouracil is indicated for palliative treatment of carcinoma of the colon, rectum, breast, stomach, and pancreas in patients considered to be incurable by surgery or other means. /Included in US product update/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1430
Fluorouracil is also indicated for treatment of bladder carcinoma, prostatic carcinoma, epithelial ovarian carcinoma, cervical carcinoma, endometrial carcinoma, anal carcinoma, esophageal carcinoma,metastatic tumors of skin carcinoma, and hepatoblastoma, and is used by intra-arterial injection for treatment of hepatic tumors and head and neck tumors. /Not included in US product label/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1430
Fluorouracil, in combination therapy, is reasonable medical therapy at some point in the management of adrenocortical carcinoma, vulvar carcinoma, penile carcinoma and carcinoid tumors (gastrointestinal and neuroendocrine tumors). /Not included in US product label/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1430
For more Therapeutic Uses (Complete) data for FLUOROURACIL (12 total), please visit the HSDB record page.
Anorexia and nausea are common adverse effects of fluorouracil, and vomiting occurs frequently. These reactions generally occur during the first week of therapy, can often be alleviated by antiemetics, and generally subside within 2 or 3 days following therapy. Stomatitis is one of the most common and often the earliest sign of specific toxicity, appearing as early as the fourth day but more commonly on the fifth to eighth day of therapy. Diarrhea, which also occurs frequently, usually appears slightly later than stomatitis, but may occur concurrently or even in the absence of stomatitis. Esophagitis, proctitis, and GI ulceration and bleeding have been reported, and paralytic ileus occurred in two patients who received excessive dosage. Patients must be closely monitored for adverse GI effects.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 1046
Leukopenia, predominantly of the granulocytopenic type, thrombocytopenia, and anemia occur commonly with fluorouracil therapy; leukopenia usually occurs after an adequate course of fluorouracil therapy. Pancytopenia and agranulocytosis also have occurred. The patient's hematologic status must be carefully monitored. The nadir of the white blood cell count usually occurs from the ninth to the fourteenth day after therapy is initiated but may occur as late as the 25th day after the first dose of fluorouracil. Maximum thrombocytopenia has been reported to occur from the seventh to seventeenth day of therapy. Hematopoietic recovery is usually rapid and by the thirtieth day, blood cell counts have usually reached the normal range.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 1047
Hair loss occurs frequently with fluorouracil therapy, and cosmetically significant alopecia has occurred in a substantial number of patients. Regrowth of hair has been reported even in patients receiving repeated courses of the drug. Partial loss of nails has occurred rarely, and diffuse melanosis of the nails has been reported. The most common type of dermatologic toxicity is a pruritic maculopapular rash which usually appears on the extremities and less frequently on the trunk. This rash is generally reversible and usually responsive to symptomatic treatment.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 1047
An erythematous, desquamative rash involving the hands and feet has been reported in patients receiving fluorouracil (in some cases, prolonged infusions of high dosages of the drug were administered). The rash may be accompanied by tingling or painful hands and feet, swollen palms and soles, and phalangeal tenderness. These adverse effects, referred to as palmar-plantar erythrodysesthesia or hand-foot syndrome, may gradually disappear over 5-7 days after discontinuance of fluorouracil therapy.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 1047
For more Drug Warnings (Complete) data for FLUOROURACIL (31 total), please visit the HSDB record page.
For the topical treatment of multiple actinic or solar keratoses. In the 5% strength it is also useful in the treatment of superficial basal cell carcinomas when conventional methods are impractical, such as with multiple lesions or difficult treatment sites. Fluorouracil injection is indicated in the palliative management of some types of cancer, including colon, esophageal, gastric, rectum, breast, biliary tract, stomach, head and neck, cervical, pancreas, renal cell, and carcinoid.
FDA Label
Fluorouracil is an antineoplastic anti-metabolite. Anti-metabolites masquerade as purine or pyrimidine - which become the building blocks of DNA. They prevent these substances from becoming incorporated into DNA during the "S" phase (of the cell cycle), stopping normal development and division. Fluorouracil blocks an enzyme which converts the cytosine nucleotide into the deoxy derivative. In addition, DNA synthesis is further inhibited because Fluorouracil blocks the incorporation of the thymidine nucleotide into the DNA strand.
Antimetabolites, Antineoplastic
Antimetabolites that are useful in cancer chemotherapy. (See all compounds classified as Antimetabolites, Antineoplastic.)
Immunosuppressive Agents
Agents that suppress immune function by one of several mechanisms of action. Classical cytotoxic immunosuppressants act by inhibiting DNA synthesis. Others may act through activation of T-CELLS or by inhibiting the activation of HELPER CELLS. While immunosuppression has been brought about in the past primarily to prevent rejection of transplanted organs, new applications involving mediation of the effects of INTERLEUKINS and other CYTOKINES are emerging. (See all compounds classified as Immunosuppressive Agents.)
Antimetabolites
Drugs that are chemically similar to naturally occurring metabolites, but differ enough to interfere with normal metabolic pathways. (From AMA Drug Evaluations Annual, 1994, p2033) (See all compounds classified as Antimetabolites.)
L01BC02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01B - Antimetabolites
L01BC - Pyrimidine analogues
L01BC02 - Fluorouracil
Absorption
28-100%
Route of Elimination
Seven percent to 20% of the parent drug is excreted unchanged in the urine in 6 hours; of this over 90% is excreted in the first hour. The remaining percentage of the administered dose is metabolized, primarily in the liver.
Given by continuous iv infusion for 24 hr, plasma concn in range of 0.5 to 3.0 uM are obtained and urinary excretion of fluorouracil is only 4%.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1229
Fluorouracil readily enters cerebrospinal fluid, and concn of about 7 uM are reached within 30 min after iv admin; values are sustained for approx 3 hr and subside slowly during period of 9 hr.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1229
Fluorouracil crosses the placenta in rats.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 1048
Following iv administration of fluorouracil, no intact drug is detected in plasma after 3 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 1048
For more Absorption, Distribution and Excretion (Complete) data for FLUOROURACIL (7 total), please visit the HSDB record page.
Hepatic. The catabolic metabolism of fluorouracil results in degradation products ( e.g., CO2, urea and -fluoro--alanine) which are inactive.
A small portion of fluorouracil is anabolized in the tissues to 5-fluoro-2'-deoxyuridine and then to 5-fluoro-2'-deoxyuridine-5'-monophosphate, the active metabolite of the drug. The major portion of the drug is degraded in the liver. The metabolites are excreted as respiratory carbon dioxide and as urea, alpha-fluoro-beta-alanine, alpha-fluoro-beta-guanidopropionic acid, and alpha-fluoro-beta-ureidopropionic acid in urine. Following a single iv dose of fluorouracil, approximately 15% of the dose is excreted in urine as intact drug within 6 hours; over 90% of this is excreted in the first hour.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 1048
... Dihydropyrimidine dehydrogenase /is/ an NADPH-requiring homodimeric protein (Mr ~210 kDa) containing FMN/FAD, and an iron-sulfur cluster in each subunit. The enzyme is located mainly in liver cytosol, where it catalyzes the reduction of 5-fluorouracil and related pyrimidines ...
Klaassen, C.D. (ed). Casarett and Doull's Toxicology. The Basic Science of Poisons. 6th ed. New York, NY: McGraw-Hill, 2001., p. 149
... Several routes are available for the formation of the 5'-monophosphate nucleotide (F-UMP) in animal cells. 5-FU may be converted to fluorouridine by uridine phosphorylase and then to F-UMP by uridine kinase, or it may react directly with 5-phosphoribosyl-1-pyrophosphate (PRPP), in a reaction catalyzed by ... orotate phosphoribosyl transferase, to form F-UMP. Many metabolic pathways are available to F-UMP, including incorporation into RNA. A reaction sequence crucial for antineoplastic activity involves reduction of the diphosphate nucleotide by the enzyme ribonucleoside diphosphate reductase to the deoxynucleotide level and the eventual formation of 5-fluoro-2'-deoxyuridine-5'-phosphate (F-dUMP). 5-FU also may be converted directly to the deoxyriboside 5-FUdR by the enzyme thymidine phosphorylase and further to F-dUMP, a potent inhibitor of thymidylate synthesis, by thymidine kinase ... The folate cofactor, 5,10-methylenetetrahydrofolate, and F-dUMP form a covalently bound ternary complex with the enzyme /thymidylate synthase/ ...
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1405
... Metabolic degradation /of 5-FU and floxuridine/ occurs in many tissues, particularly in liver. Floxuridine is converted by thymidine or deoxyuridine phosphorylases into 5-FU. 5-FU is inactivated by reduction of the pyrimidine ring; this reaction is carried out by dihydropyrimidine dehydrogenase (DPD), which is found in liver, intestinal mucosa, tumor cells, and other tissues ... Its metabolite, 5-fluoro-5,6-dihydrouracil ... is ultimately degraded to alpha-fluoro-beta-alanine ... Although the liver contains high concn of DPD, dosage does not have to be modified in patients with hepatic dysfunction, presumably because of degradation of the drug at extrahepatic sites or by vast excess of this enzyme in the liver ...
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1407
5-Fluorouracil is a known human metabolite of Tegafur.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
10-20 minutes
Following iv administration, the plasma elimination half-life averages about 16 minutes (range: 8-20 minutes) and is dose dependent.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 1048
Rapid iv admin of 5-FU produces plasma concn of 0.1 to 1.0 mM; plasma clearance is rapid (half-life 10 to 20 min) ...
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1407
The precise mechanism of action has not been fully determined, but the main mechanism of fluorouracil is thought to be the binding of the deoxyribonucleotide of the drug (FdUMP) and the folate cofactor, N510-methylenetetrahydrofolate, to thymidylate synthase (TS) to form a covalently bound ternary complex. This results in the inhibition of the formation of thymidylate from uracil, which leads to the inhibition of DNA and RNA synthesis and cell death. Fluorouracil can also be incorporated into RNA in place of uridine triphosphate (UTP), producing a fraudulent RNA and interfering with RNA processing and protein synthesis.
5-FU requires enzymatic conversion to the nucleotide (ribosylation and phosphorylation) in order to exert its cytotoxic activity. Several routes are available for the formation of the 5'-monophosphate nucleotide (F-UMP) in animal cells. 5-FU may be converted to fluorouridine by uridine phosphorylase and then to F-UMP by uridine kinase, or it may react directly with 5-phosphoribosyl-1-pyrophosphate (PRPP), in a reaction catalyzed by ... orotate phosphoribosyl transferase, to form F-UMP. Many metabolic pathways are available to F-UMP, including incorporation in to RNA. A reaction sequence crucial for antineoplastic activity involves reduction of the diphosphate nucleotide by the enzyme ribonucleoside diphosphate reductase to the deoxynucleotide level and the eventual formation of 5-fluoro-2'-deoxyuridine-5'-phosphate (F-dUMP). 5-FU also may be converted directly to the deoxyriboside 5-FUdR by the enzyme thymidine phosphorylase and further to F-dUMP, a potent inhibitor of thymidylate synthesis, by thymidine kinase ... The interaction between F-dUMP and the enzyme thymidylate synthase leads to depletion of TTP, a necessary constituent of DNA ... The folate cofactor, 5,10-methylenetetrahydrofolate, and F-dUMP form a covalently bound ternary complex with the enzyme. The inhibitory complex resembles the transition state formed during the normal enzymatic reaction when dUMP is converted to thymidylate. Although the physiological complex progresses to the synthesis of thymidylate by transfer of the methylene group and 2 hydrogen atoms from folate to dUMP, this reaction is blocked in the inhibitory complex by the stability of the fluorine carbon bond on F-dUMP; sustained inhibition of the enzyme results ...
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1405
Although the precise mechanisms of action of fluorouracil have not been fully elucidated, the main mechanism is thought to be the binding of the deoxyribonucleotide of the drug (FdUMP) and the folate cofactor, N5-10-methylenetetrahydrofolate, to thymidylate synthase (TS) to form a covalently bound ternary complex, which inhibits the formation of thymidylate from uracil, thereby interfering with DNA synthesis. In addition, FUTP can be incorporated into RNA in place of uridine triphosphate (UTP), producing a fraudulent RNA and interfering with RNA processing and protein synthesis.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 1048
Fluorouracil is an antimetabolite of the pyrimidine analog type. Fluorouracil is considered to be cell cycle-specific for the S phase of cell division. Activity results from its conversion to an active metabolite in the tissues, and includes inhibition of DNA and RNA synthesis.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 1430
There is evidence that the metabolism of fluorouracil in the anabolic pathway blocks the methylation reaction of deoxyuridylic acid to thymidylic acid. In this manner fluorouracil interferes with the synthesis of deoxyribonucleic acid (DNA) and to a lesser extent inhibits the formation of ribonucleic acid (RNA). Since DNA and RNA are essential for cell division and growth, the effect of fluorouracil may be to create a thymine deficiency which provokes unbalanced growth and death of the cell. The effects of DNA and RNA deprivation are most marked on those cells which grow more rapidly and take up fluorouracil at a more rapid rate. The catabolic metabolism of fluorouracil results in degradation products (eg, CO2 , urea, (alpha)-fluoro-(beta)-alanine) which are inactive. /Efudex Solutions/
Physicians Desk Reference 61st ed, Thomson PDR, Montvale, NJ 2007., p. 3363
For more Mechanism of Action (Complete) data for FLUOROURACIL (7 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
22
PharmaCompass offers a list of Fluorouracil API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Fluorouracil manufacturer or Fluorouracil supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Fluorouracil manufacturer or Fluorouracil supplier.
PharmaCompass also assists you with knowing the Fluorouracil API Price utilized in the formulation of products. Fluorouracil API Price is not always fixed or binding as the Fluorouracil Price is obtained through a variety of data sources. The Fluorouracil Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Fluorouracil manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Fluorouracil, including repackagers and relabelers. The FDA regulates Fluorouracil manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Fluorouracil API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Fluorouracil manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Fluorouracil supplier is an individual or a company that provides Fluorouracil active pharmaceutical ingredient (API) or Fluorouracil finished formulations upon request. The Fluorouracil suppliers may include Fluorouracil API manufacturers, exporters, distributors and traders.
click here to find a list of Fluorouracil suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Fluorouracil DMF (Drug Master File) is a document detailing the whole manufacturing process of Fluorouracil active pharmaceutical ingredient (API) in detail. Different forms of Fluorouracil DMFs exist exist since differing nations have different regulations, such as Fluorouracil USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Fluorouracil DMF submitted to regulatory agencies in the US is known as a USDMF. Fluorouracil USDMF includes data on Fluorouracil's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Fluorouracil USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Fluorouracil suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Fluorouracil Drug Master File in Japan (Fluorouracil JDMF) empowers Fluorouracil API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Fluorouracil JDMF during the approval evaluation for pharmaceutical products. At the time of Fluorouracil JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Fluorouracil suppliers with JDMF on PharmaCompass.
A Fluorouracil CEP of the European Pharmacopoeia monograph is often referred to as a Fluorouracil Certificate of Suitability (COS). The purpose of a Fluorouracil CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Fluorouracil EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Fluorouracil to their clients by showing that a Fluorouracil CEP has been issued for it. The manufacturer submits a Fluorouracil CEP (COS) as part of the market authorization procedure, and it takes on the role of a Fluorouracil CEP holder for the record. Additionally, the data presented in the Fluorouracil CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Fluorouracil DMF.
A Fluorouracil CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Fluorouracil CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Fluorouracil suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Fluorouracil as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Fluorouracil API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Fluorouracil as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Fluorouracil and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Fluorouracil NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Fluorouracil suppliers with NDC on PharmaCompass.
Fluorouracil Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Fluorouracil GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Fluorouracil GMP manufacturer or Fluorouracil GMP API supplier for your needs.
A Fluorouracil CoA (Certificate of Analysis) is a formal document that attests to Fluorouracil's compliance with Fluorouracil specifications and serves as a tool for batch-level quality control.
Fluorouracil CoA mostly includes findings from lab analyses of a specific batch. For each Fluorouracil CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Fluorouracil may be tested according to a variety of international standards, such as European Pharmacopoeia (Fluorouracil EP), Fluorouracil JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Fluorouracil USP).
