
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
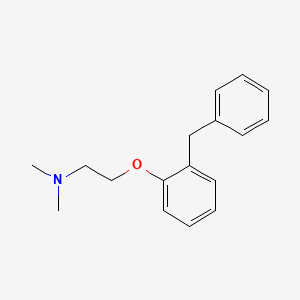

1. N,n-dimethyl-2-(alpha-phenyl-o-tolyloxy)ethylamine
2. Phenyltoloxamine Hydrochloride
1. 92-12-6
2. Phenoxadrin
3. Phenoxadrine
4. Phentoloxamine
5. Bistrimin
6. Bristamin
7. Histionex
8. Antin
9. 2-(2-benzylphenoxy)-n,n-dimethylethanamine
10. 2-(2-dimethylaminoethoxy)diphenylmethane
11. Phenyltoloxamine Resin Complex
12. Chembl186720
13. N,n-dimethyl-2-(a-phenyl-o-toloxy)ethylamine
14. K65lb6598j
15. Ethanamine, N,n-dimethyl-2-(2-(phenylmethyl)phenoxy)-
16. Ethanamine, N,n-dimethyl-2-[2-(phenylmethyl)phenoxy]-
17. Antin; Bistrimin; Bristamin; C 5581h; Histionex
18. Feniltoloxamina
19. Feniltolossamina
20. Feniltolossamina [dcit]
21. Phenyltoloxaminum
22. Phenyltoloxamine [inn:ban]
23. Feniltoloxamina [inn-spanish]
24. Phenyltoloxaminum [inn-latin]
25. Einecs 202-127-9
26. C 5581h
27. Brn 1986598
28. N,n-dimethyl-2-(alpha-phenyl-o-tolyloxy)ethylamine
29. Fenoxadrin
30. Phentoloxamin
31. Unii-k65lb6598j
32. N,n-dimethyl-2-(2-(phenylmethyl)phenoxy)ethanamine
33. Ethanamine, N,n-dimethyl-2-((alpha-phenyl-o-tolyl)oxy)-
34. Floxamine (salt/mix)
35. Schembl28854
36. Phenyltoloxamine [mi]
37. 4-06-00-04630 (beilstein Handbook Reference)
38. Phenyltoloxamine [inn]
39. Zinc1931
40. Phenyltoloxamine [vandf]
41. Dtxsid9023467
42. Phenyltoloxamine [who-dd]
43. Chebi:135047
44. Hy-b1733
45. Bdbm50151046
46. Db11160
47. Dimethyl{2-[2-benzylphenoxy]ethyl}amine
48. Ncgc00021141-01
49. Ncgc00021141-02
50. Ncgc00021141-03
51. Ncgc00022229-03
52. C 5581 H
53. 2-(2-benzylphenoxy)-n,n-dimethylethylamine
54. 2-[2-(dimethylamino)ethoxy]diphenylmethane
55. O-benzylphenyl 2-(dimethylamino)ethyl Ether
56. 2-(2-benzylphenoxy)-n,n-dimethyl-ethanamine
57. Cs-0013741
58. [2-(2-benzyl-phenoxy)-ethyl]-dimethyl-amine
59. Phenyltoloxamine Resin Complex [vandf]
60. 2-(2-benzylphenoxy)-n,n-dimethylethanamine #
61. 2-benzylphenyl-.beta.-dimethylamino Ethyl Ether
62. Phenyltoloxamine Resin Complex [who-dd]
63. N,n-dimethyl-2-(.alpha.-phenyl-o-toloxy)ethylamine
64. Q7181443
65. N,n-dimethyl-2-((.alpha.-phenyl-o-tolyl)oxy)ethylamine
66. N,n-dimethyl-2-{[2-(phenylmethyl)phenyl]oxy}ethanamine
67. Ethylamine, N,n-dimethyl-2-[(.alpha.-phenyl-o-tolyl)oxy]-
68. N,n-dimethyl-2-(.alpha.-phenyl-o-tolyloxy)ethylamine
| Molecular Weight | 255.35 g/mol |
|---|---|
| Molecular Formula | C17H21NO |
| XLogP3 | 3.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 6 |
| Exact Mass | 255.162314293 g/mol |
| Monoisotopic Mass | 255.162314293 g/mol |
| Topological Polar Surface Area | 12.5 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 235 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
The primary therapeutic use for which phenyltoloxamine is currently indicated is as an adjuvant therapy in various combination products containing an analgesic(s) (either narcotic or non-narcotic), where it is expected to potentiate the pain relieving, anti-tussive, etc. effect(s) of the analgesic component of the product. In that regard, some of these aforementioned combination products are typically indicated for the temporary relief of minor aches and pains like headache, muscular aches, backaches, minor arthritis pain, common cold, toothaches, menstrual cramps, etc; or perhaps for the treatment of exhausting or non-productive cough, associated with cold or with upper respiratory allergic condition that does not respond to non-narcotic antitussives.
As a member of the first generation H1 antihistamines, it is known that phenyltoloxamine - like virtually all first generation H1 antihistamines - has a propensity for crossing the blood-brain barrier and acting on H1 histamine receptors there to interfere with neurotransmission. The most common results of this kind of first generation H1 antihistamine CNS neurotransmission interference are adverse effects like drowsiness, sedation, somnolence, and fatigue. Given these effects, under specific circumstances like a patient experiencing a pain or a cough that may be preoccupying all of their waking energy and attention, it is perhaps possible that the sedative and tranquilizing characteristics of phenyltoloxamine may be the factors that contribute to its apparent adjunctive analgesic and antitussive actions.
Absorption
Readily accessible data regarding the absorption of phenyltoloxamine is not available. In fact, many first-generation H1 antihistamines have never had their pharmacokinetics (ie. absorption, distribution, metabolism, and elimination) optimally investigated.
Route of Elimination
Readily accessible data regarding the primary route of elimination of phenyltoloxamine is not available. In fact, many first-generation H1 antihistamines have never had their pharmacokinetics (ie. absorption, distribution, metabolism, and elimination) optimally investigated.
Volume of Distribution
Readily accessible data regarding the volume of distribution of phenyltoloxamine is not available. In fact, many first-generation H1 antihistamines have never had their pharmacokinetics (ie. absorption, distribution, metabolism, and elimination) optimally investigated.
Clearance
Readily accessible data regarding the clearance of phenyltoloxamine is not available. In fact, many first-generation H1 antihistamines have never had their pharmacokinetics (ie. absorption, distribution, metabolism, and elimination) optimally investigated.
Readily accessible data regarding the metabolism of phenyltoloxamine is not available. In fact, many first-generation H1 antihistamines have never had their pharmacokinetics (ie. absorption, distribution, metabolism, and elimination) optimally investigated.
Readily accessible data regarding the half-life of phenyltoloxamine is not available. In fact, many first-generation H1 antihistamines have never had their pharmacokinetics (ie. absorption, distribution, metabolism, and elimination) optimally investigated.
As a first-generation H1 antihistamine, phenyltoloxamine interferes with the agonist activity of histamine at the H1 receptor and are ostensibly used to attenuate inflammatory processes as a means to treat conditions like allergic rhinitis, allergic conjunctivitis, and urticaria. Reduction of the activity of the NF-kB (nuclear factor kappa-light-chain-enhancer of activated B cells) immune response transcription factor via the phospholipase C and phosphatidylinositol (PIP2) signaling pathways also serves to decrease antigen presentation and the expression of pro-inflammatory cytokines, cell adhesion molecules, and chemotactic factors. Moreover, lowering calcium ion concentration leads to increased mast cell stability which reduces further histamine release. Additionally, first-generation antihistamines like phenyltoloxamine readily cross the blood-brain barrier and cause sedation and other adverse central nervous system (CNS) effects, like nervousness and insomnia. By comparison, second-generation antihistamines are more selective for H1 receptors in the peripheral nervous system and do not cross the blood-brain barrier, resulting in fewer adverse drug effects overall. Furthermore, although some studies propose that phenyltoloxamine may possess some intrinsic antispasmodic and distinct local anesthetic properties, the specific mechanisms of action for these effects have not been formalized. Also, even though the combination of phenyltoloxamine's ability to cross the blood-brain barrier and cause various tranquilizing effects may explain to some extent how it may be able to potentiate analgesic effects, there are also studies that observed no potentiating effects associated with phenyltoloxamine use either.
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
28
PharmaCompass offers a list of Phenyltoloxamine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Phenyltoloxamine manufacturer or Phenyltoloxamine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Phenyltoloxamine manufacturer or Phenyltoloxamine supplier.
PharmaCompass also assists you with knowing the Phenyltoloxamine API Price utilized in the formulation of products. Phenyltoloxamine API Price is not always fixed or binding as the Phenyltoloxamine Price is obtained through a variety of data sources. The Phenyltoloxamine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Feniltoloxamina manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Feniltoloxamina, including repackagers and relabelers. The FDA regulates Feniltoloxamina manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Feniltoloxamina API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Feniltoloxamina supplier is an individual or a company that provides Feniltoloxamina active pharmaceutical ingredient (API) or Feniltoloxamina finished formulations upon request. The Feniltoloxamina suppliers may include Feniltoloxamina API manufacturers, exporters, distributors and traders.
Feniltoloxamina Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Feniltoloxamina GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Feniltoloxamina GMP manufacturer or Feniltoloxamina GMP API supplier for your needs.
A Feniltoloxamina CoA (Certificate of Analysis) is a formal document that attests to Feniltoloxamina's compliance with Feniltoloxamina specifications and serves as a tool for batch-level quality control.
Feniltoloxamina CoA mostly includes findings from lab analyses of a specific batch. For each Feniltoloxamina CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Feniltoloxamina may be tested according to a variety of international standards, such as European Pharmacopoeia (Feniltoloxamina EP), Feniltoloxamina JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Feniltoloxamina USP).
