


1. Calcium Mefolinate
2. 26560-38-3
3. Calcium Methyltetrahydrofolate
4. 9i291hq60g
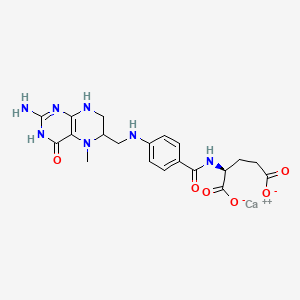
5. Calcium;(2s)-2-[[4-[(2-amino-5-methyl-4-oxo-3,6,7,8-tetrahydropteridin-6-yl)methylamino]benzoyl]amino]pentanedioate
6. 5-methyltetrahydrofolic Acid Calcium Salt
7. Unii-9i291hq60g
8. Nsc 173328
9. Nsc-173328
10. Calcium (2s)-2-(4-(((2-amino-5-methyl-4-oxo-3,4,5,6,7,8-hexahydropteridin-6-yl)methyl)amino)benzamido)pentanedioate
11. Calciumn5-methyltetrahydrofolate
12. Calcium Mefolinate [who-dd]
13. Calcium D,l-5-methyltetrahydrofolate
14. Akos015901141
15. As-75127
16. Hy-17557
17. L-glutamic Acid, N-(4-(((2-amino-1,4,5,6,7,8-hexahydro-5-methyl-4-oxo-6-pteridinyl)methyl)amino)benzoyl)-, Calcium Salt (1:1)
18. N-[4-[[(2-amino-1,4,5,6,7,8-hexahydro-5-methyl-4-oxo-6-pteridinyl)methyl]amino]benzoyl]-l-glutamic Acid
19. 5-methyltetrahydrofolic Acid Calcium Salt Hydrate
20. Calcium D,l-5-methyltetrahydrofolate [usp-rs]
21. Q27272582
22. Calcium (2s)-2-[(4-{[(2-amino-5-methyl-4-oxo-3,4,5,6,7,8-hexahydropteridin-6-yl)methyl]amino}phenyl)formamido]pentanedioate
23. Calcium (4-(((2-amino-5-methyl-4-oxo-3,4,5,6,7,8-hexahydropteridin-6-yl)methyl)amino)benzoyl)-l-glutamate
| Molecular Weight | 497.5 g/mol |
|---|---|
| Molecular Formula | C20H23CaN7O6 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 7 |
| Exact Mass | 497.1335723 g/mol |
| Monoisotopic Mass | 497.1335723 g/mol |
| Topological Polar Surface Area | 204 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 854 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |