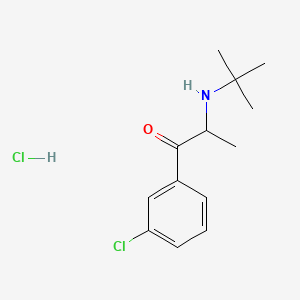

1. (+-)-1-(3-chlorophenyl)-2-((1,1-dimethylethyl)amino)-1-propanone
2. Amfebutamone
3. Bupropion
4. Bupropion Hydrochloride, (+-)-isomer
5. Bupropion, (+-)-isomer
6. Quomen
7. Wellbutrin
8. Zyban (anti-smoking)
9. Zyban (bupropion)
10. Zyntabac
1. 31677-93-7
2. Bupropion Hcl
3. 2-(tert-butylamino)-1-(3-chlorophenyl)propan-1-one Hydrochloride
4. Wellbutrin
5. Amfebutamone Hydrochloride
6. Zyban
7. Wellbutrin Xl
8. Wellbutrin Sr
9. Budeprion
10. Forfivo Xl
11. Wellbatrin
12. Amfebutamone (bupropion) Hcl
13. Bw 323
14. Mysimba
15. Bupropion (hydrochloride)
16. Bupropion Sr
17. 2-(tert-butylamino)-3'-chloropropiophenone Hydrochloride
18. Zg7e5poy8o
19. Nsc-315851
20. 31677-93-7 (hcl)
21. Elontril
22. Bw-323
23. 234447-17-7
24. 2-(tert-butylamino)-1-(3-chlorophenyl)propan-1-one;hydrochloride
25. 1-(3-chlorophenyl)-2-[(1,1-dimethylethyl)amino]-1-propanone Hydrochloride
26. 1-propanone, 1-(3-chlorophenyl)-2-[(1,1-dimethylethyl)amino]-, Hydrochloride (1:1), (-)-
27. Dsstox_cid_24561
28. Dsstox_rid_80314
29. Dsstox_gsid_44561
30. Bupropion Hydrochloride 100 Microg/ml In Acetonitrile
31. Bupropion Hydrocloride
32. 2-(tert-butylamino)-1-(3-chlorophenyl)propan-1-one Hcl
33. Zyban (pharmaceutical)
34. Voxra
35. Budeprion Xl
36. (+/-)-bupropion; Amfebutamon; Amfebutamone; Aplenzin; Bupropion Sr; Bupropion Xl
37. Smr000058423
38. Budeprion Xl 300
39. Nsc315851
40. Amfebutamon Hydrochloride
41. Sr-01000002989
42. Einecs 250-759-9
43. Unii-zg7e5poy8o
44. Nsc 315851
45. Quomem
46. Bupropion Hydrochloride [usan]
47. Wellbutrin Xr
48. Wellbutrin Retard
49. Wellbutrin (tn)
50. Bupropion D9 Hcl
51. Prestwick_668
52. Einecs 252-243-9
53. Mfcd00055209
54. Zyban (tn)
55. M-chloro-alpha-tert-butylaminopropiophenone Hydrochloride
56. Alpha-(tert-butylamino)-m-chloropropiophenone Hydrochloride
57. M-chloro-alpha-tert-butylaminopropionphenone Hydrochloride
58. Bupropion Hydrochloride [usan:usp:jan]
59. (+-)-2-(tert-butylamino)-3'-chloropropiophenone Hydrochloride
60. (+-)-alpha-tert-butylamino-3-chloropropiophenone Hydrochloride
61. Amfebutamone (bupropion)
62. Ncgc00016807-01
63. Cas-31677-93-7
64. Chembl1698
65. Schembl41602
66. Mls000069376
67. Mls001401370
68. Mls002320678
69. Spectrum1504174
70. Bupropion Hydrochloride- Bio-x
71. Bupropion Hydrochloride Solution
72. Chebi:3220
73. Dtxsid6044561
74. Hy-b0403a
75. Bvf 033
76. Bw 322u
77. Cpi-300
78. Hms1568b20
79. Hms1922f09
80. Pharmakon1600-01504174
81. Bupropion Hydrochloride (jan/usp)
82. Bcp22394
83. Bupropion Hydrochloride [mi]
84. Tox21_110621
85. Tox21_301844
86. Tox21_500166
87. Ac-196
88. Amfebutamone-hydrochloride(bupropion)
89. Bupropion Hydrochloride [jan]
90. Bw-323u66
91. Ccg-39085
92. Nsc758686
93. Propiophenone, 2-(tert-butylamino)-3'-chloro-, Hydrochloride, (+-)-
94. Bupropion Hydrochloride [hsdb]
95. Akos015844544
96. Tox21_110621_1
97. Ab02273
98. Bcp9000462
99. Bupropion Hydrochloride [mart.]
100. Bupropion Hydrochloride [vandf]
101. Ccg-100911
102. Ks-1034
103. Lp00166
104. Nc00161
105. 1-propanone, 1-(3-chlorophenyl)-2-((1,1-dimethylethyl)amino)-, Hydrochloride, (+-)-
106. Bupropion Hydrochloride [usp-rs]
107. Bupropion Hydrochloride [who-dd]
108. Ncgc00015122-11
109. Ncgc00093650-01
110. Ncgc00093650-02
111. Ncgc00093650-03
112. Ncgc00093650-04
113. Ncgc00093650-05
114. Ncgc00180895-01
115. Ncgc00180895-02
116. Ncgc00255927-01
117. Ncgc00260851-01
118. Propiophenone, Hydrochloride, (.+-.)-
119. Bb164268
120. Smr001338824
121. Bcp0726000049
122. Am20060780
123. B-102
124. B3649
125. Bupropion Hydrochloride [orange Book]
126. Eu-0100166
127. S2452
128. Sw196823-6
129. Bupropion Hydrochloride [usp Impurity]
130. 77b937
131. Bupropion Hydrochloride [usp Monograph]
132. D00817
133. Bupropion Hydrochloride, >=98% (hplc), Solid
134. Contrave Component Bupropion Hydrochloride
135. A820950
136. Bupropion Hydrochloride Component Of Contrave
137. Sr-01000002989-2
138. Q27295463
139. Sr-01000002989-10
140. (+/-)-2-(t-butylamino)-3'-chloropropiophenone Hydrochloride
141. 1-propanone,1-dimethylethyl)amino]-, Hydrochloride, (.+-.)-
142. Bupropion Hydrochloride 1.0 Mg/ml In Methanol (as Free Base)
143. (+/-)-2-(tert-butylamino)-3'-chloropropiophenone Hydrochloride
144. (.+-.)-2-(tert-butylamino)-3'-chloropropiophenone Hydrochloride
145. 2-(tert-butylamino)-1-(3-chlorophenyl)-1-propanone Hydrochloride
146. Bupropion Hydrochloride, United States Pharmacopeia (usp) Reference Standard
147. 1-(3-chlorophenyl)-2-[(1,1-dimethylethyl)amino]-1-propane, Monohydrochloride
148. 1-propanone, 1-(3-chlorophenyl)-2-[(1,1-dimethylethyl)amino]-, Hydrochloride (1:1)
149. 2-(tert-butylamino)-1-(3-chlorophenyl)propan-1-one Hydrochloride;bupropion Hcl
150. Bupropion Hydrochloride, Pharmaceutical Secondary Standard; Certified Reference Material
151. ( Inverted Question Mark)-1-(3-chlorophenyl)-2-[(1,1-dimethylethyl)amino]-1-propanone Hydrochloride
152. 1-propanone, 1-(3-chlorophenyl)-2-((1,1-dimethylethyl)amino)-, Hydrochloride, (+/-)-
153. Bupropion Hydrochloride Solution, 1.0 Mg/ml In Methanol (as Free Base), Ampule Of 1 Ml, Certified Reference Material
1. Amfebutamon
2. Amfebutamona
3. Amfebutamonum
4. Aplenzin
5. Bupropion
| Molecular Weight | 276.20 g/mol |
|---|---|
| Molecular Formula | C13H19Cl2NO |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 275.0843696 g/mol |
| Monoisotopic Mass | 275.0843696 g/mol |
| Topological Polar Surface Area | 29.1 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 247 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 12 | |
|---|---|
| Drug Name | Bupropion hydrochloride |
| PubMed Health | Bupropion (By mouth) |
| Drug Classes | Antidepressant, Smoking Cessation Agent |
| Drug Label | hydrochloride. The molecular weight is 276.2. The molecular formula is C13H18ClNOHCl. Bupropion hydrochloride powder is white, crystalline, and highly soluble in water. It has a bitter taste and produces the sensation of local anesthesia on the oral... |
| Active Ingredient | Bupropion hydrochloride |
| Dosage Form | Tablet, extended release; Tablet |
| Route | Oral |
| Strength | 300mg; 75mg; 200mg; 100mg; 150mg |
| Market Status | Prescription |
| Company | Watson Labs; Wockhardt; Anchen Pharms; Jubilant Generics; Actavis Labs Fl; Apotex; Zydus Pharms Usa; Sandoz; Sun Pharma Global; Mylan; Actavis; Impax Labs |
| 2 of 12 | |
|---|---|
| Drug Name | Forfivo xl |
| Drug Label | FORFIVO XL (bupropion hydrochloride), an antidepressant of the aminoketone class, is chemically unrelated to tricyclic, tetracyclic, selective serotonin re-uptake inhibitor, or other known antidepressant agents. Its structure closely resembles that o... |
| Active Ingredient | Bupropion hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 450mg |
| Market Status | Prescription |
| Company | Edgemont Pharms |
| 3 of 12 | |
|---|---|
| Drug Name | Wellbutrin |
| PubMed Health | Bupropion (By mouth) |
| Drug Classes | Antidepressant, Smoking Cessation Agent |
| Drug Label | DESCRIPTIONWELLBUTRINXL (bupropion hydrochloride), an antidepressant of the aminoketone class, is chemically unrelated to tricyclic, tetracyclic, selective serotonin re-uptake inhibitor, or other known antidepressant agents. Its structure closely r... |
| Active Ingredient | Bupropion hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 75mg; 100mg |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 4 of 12 | |
|---|---|
| Drug Name | Wellbutrin sr |
| PubMed Health | Bupropion (By mouth) |
| Drug Classes | Antidepressant, Smoking Cessation Agent |
| Drug Label | WELLBUTRINSR (bupropion hydrochloride), an antidepressant of the aminoketone class, is chemically unrelated to tricyclic, tetracyclic, selective serotonin reuptake inhibitor, or other known antidepressant agents. Its structure closely resembles... |
| Active Ingredient | Bupropion hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 200mg; 150mg; 100mg |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 5 of 12 | |
|---|---|
| Drug Name | Wellbutrin xl |
| Drug Label | DESCRIPTIONWELLBUTRINXL (bupropion hydrochloride), an antidepressant of the aminoketone class, is chemically unrelated to tricyclic, tetracyclic, selective serotonin re-uptake inhibitor, or other known antidepressant agents. Its structure closely r... |
| Active Ingredient | Bupropion hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 150mg; 300mg |
| Market Status | Prescription |
| Company | Valeant Intl |
| 6 of 12 | |
|---|---|
| Drug Name | Zyban |
| PubMed Health | Bupropion (By mouth) |
| Drug Classes | Antidepressant, Smoking Cessation Agent |
| Drug Label | ZYBAN (bupropion hydrochloride) SustainedRelease Tablets are a nonnicotine aid to smoking cessation. ZYBAN is chemically unrelated to nicotine or other agents currently used in the treatment of nicotine addiction. Initially developed and market... |
| Active Ingredient | Bupropion hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 150mg |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 7 of 12 | |
|---|---|
| Drug Name | Bupropion hydrochloride |
| PubMed Health | Bupropion (By mouth) |
| Drug Classes | Antidepressant, Smoking Cessation Agent |
| Drug Label | hydrochloride. The molecular weight is 276.2. The molecular formula is C13H18ClNOHCl. Bupropion hydrochloride powder is white, crystalline, and highly soluble in water. It has a bitter taste and produces the sensation of local anesthesia on the oral... |
| Active Ingredient | Bupropion hydrochloride |
| Dosage Form | Tablet, extended release; Tablet |
| Route | Oral |
| Strength | 300mg; 75mg; 200mg; 100mg; 150mg |
| Market Status | Prescription |
| Company | Watson Labs; Wockhardt; Anchen Pharms; Jubilant Generics; Actavis Labs Fl; Apotex; Zydus Pharms Usa; Sandoz; Sun Pharma Global; Mylan; Actavis; Impax Labs |
| 8 of 12 | |
|---|---|
| Drug Name | Forfivo xl |
| Drug Label | FORFIVO XL (bupropion hydrochloride), an antidepressant of the aminoketone class, is chemically unrelated to tricyclic, tetracyclic, selective serotonin re-uptake inhibitor, or other known antidepressant agents. Its structure closely resembles that o... |
| Active Ingredient | Bupropion hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 450mg |
| Market Status | Prescription |
| Company | Edgemont Pharms |
| 9 of 12 | |
|---|---|
| Drug Name | Wellbutrin |
| PubMed Health | Bupropion (By mouth) |
| Drug Classes | Antidepressant, Smoking Cessation Agent |
| Drug Label | DESCRIPTIONWELLBUTRINXL (bupropion hydrochloride), an antidepressant of the aminoketone class, is chemically unrelated to tricyclic, tetracyclic, selective serotonin re-uptake inhibitor, or other known antidepressant agents. Its structure closely r... |
| Active Ingredient | Bupropion hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 75mg; 100mg |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 10 of 12 | |
|---|---|
| Drug Name | Wellbutrin sr |
| PubMed Health | Bupropion (By mouth) |
| Drug Classes | Antidepressant, Smoking Cessation Agent |
| Drug Label | WELLBUTRINSR (bupropion hydrochloride), an antidepressant of the aminoketone class, is chemically unrelated to tricyclic, tetracyclic, selective serotonin reuptake inhibitor, or other known antidepressant agents. Its structure closely resembles... |
| Active Ingredient | Bupropion hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 200mg; 150mg; 100mg |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 11 of 12 | |
|---|---|
| Drug Name | Wellbutrin xl |
| Drug Label | DESCRIPTIONWELLBUTRINXL (bupropion hydrochloride), an antidepressant of the aminoketone class, is chemically unrelated to tricyclic, tetracyclic, selective serotonin re-uptake inhibitor, or other known antidepressant agents. Its structure closely r... |
| Active Ingredient | Bupropion hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 150mg; 300mg |
| Market Status | Prescription |
| Company | Valeant Intl |
| 12 of 12 | |
|---|---|
| Drug Name | Zyban |
| PubMed Health | Bupropion (By mouth) |
| Drug Classes | Antidepressant, Smoking Cessation Agent |
| Drug Label | ZYBAN (bupropion hydrochloride) SustainedRelease Tablets are a nonnicotine aid to smoking cessation. ZYBAN is chemically unrelated to nicotine or other agents currently used in the treatment of nicotine addiction. Initially developed and market... |
| Active Ingredient | Bupropion hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 150mg |
| Market Status | Prescription |
| Company | Glaxosmithkline |
Mysimba is indicated, as an adjunct to a reduced-calorie diet and increased physical activity, for the management of weight in adult patients (18 years) with an initial Body Mass Index (BMI) of
- 30 kg/m2 (obese), or
- 27 kg/m2 to < 30 kg/m2 (overweight) in the presence of one or more weight-related co morbidities (e. g. , type 2 diabetes, dyslipidaemia, or controlled hypertension)
Treatment with Mysimba should be discontinued after 16 weeks if patients have not lost at least 5% of their initial body weight.
Antidepressive Agents, Second-Generation
A structurally and mechanistically diverse group of drugs that are not tricyclics or monoamine oxidase inhibitors. The most clinically important appear to act selectively on serotonergic systems, especially by inhibiting serotonin reuptake. (See all compounds classified as Antidepressive Agents, Second-Generation.)
Smoking Cessation Agents
Substances that facilitate the cessation of tobacco smoking. (See all compounds classified as Smoking Cessation Agents.)
Cytochrome P-450 CYP2D6 Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP2D6. (See all compounds classified as Cytochrome P-450 CYP2D6 Inhibitors.)
Dopamine Uptake Inhibitors
Drugs that block the transport of DOPAMINE into axon terminals or into storage vesicles within terminals. Most of the ADRENERGIC UPTAKE INHIBITORS also inhibit dopamine uptake. (See all compounds classified as Dopamine Uptake Inhibitors.)
A08AA