

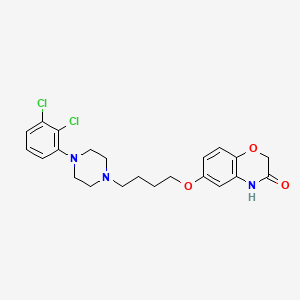

1. Brilaroxazine
2. 1239729-06-6
3. X8l60ba01i
4. 6-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2h-benzo[b][1,4]oxazin-3(4h)-one
5. 2h-1,4-benzoxazin-3(4h)-one, 6-(4-(4-(2,3-dichlorophenyl)-1-piperazinyl)butoxy)-
6. 2h-1,4-benzoxazin-3(4h)-one, 6-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy]-
7. 6-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2h-benzo(b)(1,4)oxazin-3(4h)-one
8. Brilaroxazin
9. Brilaroxazine [inn]
10. Unii-x8l60ba01i
11. Brilaroxazine [who-dd]
12. Schembl3404085
13. Bdbm312188
14. Dtxsid001136921
15. Db09226
16. Rp-5063
17. Us9604944, 15b (example 14)
18. Hy-109112
19. Cs-0077721
20. 6-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy]-2h-1,4-benzoxazin-3(4h)-one
21. 6-[4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy]-4h-1,4-benzoxazin-3-one
22. 2036070-48-9
| Molecular Weight | 450.4 g/mol |
|---|---|
| Molecular Formula | C22H25Cl2N3O3 |
| XLogP3 | 4.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 7 |
| Exact Mass | 449.1272971 g/mol |
| Monoisotopic Mass | 449.1272971 g/mol |
| Topological Polar Surface Area | 54 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 562 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for the treatment of schizophrenia and schizoaffective disorder.