
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
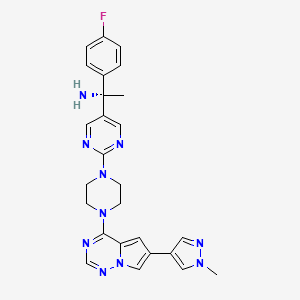

1. (1s)-1-(4-fluorophenyl)-1-(2-(4-(6-(1-methyl-1h-pyrazol-4-yl)pyrrolo(2,1-f)(1,2,4)triazin-4-yl)-1-piperazinyl)-5-pyrimidinyl)ethanamine
2. 5-pyrimidinemethanamine, Alpha-(4-fluorophenyl)-alpha-methyl-2-(4-(6-(1-methyl-1h-pyrazol-4-yl)pyrrolo(2,1-f)(1,2,4)triazin-4-yl)-1-piperazinyl)-, (alphas)-
3. Ayvakit
4. Blu-285
1. Blu-285
2. 1703793-34-3
3. Ayvakit
4. Avapritinib [inn]
5. Blu285
6. (1s)-1-(4-fluorophenyl)-1-[2-[4-[6-(1-methylpyrazol-4-yl)pyrrolo[2,1-f][1,2,4]triazin-4-yl]piperazin-1-yl]pyrimidin-5-yl]ethanamine
7. 513p80b4yj
8. C-366
9. 70c366
10. X720776
11. X-720776
12. Ayvakyt
13. Mfcd31544325
14. Ayvakit (tn)
15. Avapritinib [mi]
16. Avapritinib (usan/inn)
17. Avapritinib [usan]
18. Avapritinib (blu-285)
19. Blu-285 (avapritinib)
20. Avapritinib [who-dd]
21. Unii-513p80b4yj
22. Chembl4204794
23. Schembl16652297
24. Gtpl10368
25. Avapritinib [orange Book]
26. Blu 285
27. Bdbm469269
28. Dtxsid301027935
29. Amy16753
30. Ex-a1366
31. Us10807985, Compound 44
32. Nsc801082
33. S8553
34. Akos037648993
35. Ccg-269677
36. Cs-7577
37. Db15233
38. Nsc-801082
39. (s)-1-(4-fluorophenyl)-1-(2-(4-(6-(1-methyl-1h-pyrazol-4-yl)pyrrolo[2,1-f][1,2,4]triazin-4-yl)piperazin-1-yl)pyrimidin-5-yl)ethan-1-amine
40. Ac-31598
41. Bb166456
42. Bs-16206
43. Hy-101561
44. D11279
45. Q29213676
46. (1s)-1-(4-fluorophenyl)-1-(2-(4-(6-(1-methyl-1h-pyrazol-4-yl)pyrrolo(2,1-f)(1,2,4)triazin-4-yl)-1-piperazinyl)-5-pyrimidinyl)ethanamine
47. (1s)-1-(4-fluorophenyl)-1-(2-(4-(6-(1-methyl-1h-pyrazol-4-yl)pyrrolo(2,1-f)(1,2,4)triazin-4-yl)piperazin-1-yl)pyrimidin-5-yl)ethan-1-amine
48. (s)-1-(4-fluorophenyl)-1-(2-(4-(6-(1-methyl-1h-pyrazol-4-yl)pyrrolo(2,1-f)(1,2,4)triazin-4-yl)piperazin-yl)pyrimidin-5-yl)ethan-1-amine
49. (s)-1-(4-fluorophenyl)-1-(2-(4-(6-(1-methyl-1h-pyrazol-4-yl)pyrrolo[2,1-f][1,2,4]triazin-4-yl)piperazin-1-yl)pyrimidin-5-yl)ethanamine
50. 5-pyrimidinemethanamine, .alpha.-(4-fluorophenyl)-.alpha.-methyl-2-(4-(6-(1-methyl-1h-pyrazol-4-yl)pyrrolo(2,1-f)(1,2,4)triazin-4-yl)-1-piperazinyl)-, (.alpha.s)-
| Molecular Weight | 498.6 g/mol |
|---|---|
| Molecular Formula | C26H27FN10 |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 5 |
| Exact Mass | 498.24041907 g/mol |
| Monoisotopic Mass | 498.24041907 g/mol |
| Topological Polar Surface Area | 106 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 752 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Avapritinib is indicated for the treatment of unresectable, metastatic gastrointestinal stromal tumors with a platelet-derived growth factor receptor alpha exon 18 mutation.
Treatment of mastocytosis
Treatment of all conditions included in the category of malignant neoplasms (except haematopoietic and lymphoid tissue neoplasms)
Ayvakyt is indicated as monotherapy for the treatment of adult patients with unresectable or metastatic gastrointestinal stromal tumours (GIST) harbouring the platelet-derived growth factor receptor alpha (PDGFRA) D842V mutation.
Avapritinib is a selective kinase inhibitor that negatively modulates the action of cell transporters to resensitize them to other chemotherapies. It has a long duration of action as it is given once daily. Patients should be counselled regarding the risk of intracranial hemorrhage, CNS effects, and embryo-fetal toxicity.
L01EX18
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EX - Other protein kinase inhibitors
L01EX18 - Avapritinib
Absorption
A 300mg oral dose of avapritinib reaches a Cmax of 813ng/mL with a Tmax of 2.0-4.1h and an AUC of 15400h\*ng/mL.
Route of Elimination
Avapritinib is 70% eliminated in the feces with 11% as the unchanged drug and 18% eliminated in the urine with 0.23% as the unchanged drug.
Volume of Distribution
The mean apparent volume of distribution is 1200L.
Clearance
The mean apparent oral clearance of avapritinib is 19.5L/h.
Avapritinib is metabolized mainly by CYP3A4 and CYP2C9 in vitro. A 310mg oral dose is recovered as 49% unchanged drug, 35% hydroxy glucuronide metabolite, and 14% oxidatively deaminated metabolite.
The half life of avapritinib is 32-57h.
Avapritinib has a negative modulating effect on the transporters ABCB1 and ABCG2, which mediate the multidrug resistance phenotype of some cancers. This modulation may be due to interactions of avapritinib with the drug binding pocket of these transporters. Negative modulation of these transporters, resensitizes cancerous cells to treatment with chemotherapeutic agents like [paclitaxel].

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Through the acquisition of Avapritinib, a small molecule targeting KIT/PDGFA receptor, the deal aims to advance precision medicine in oncology.
Lead Product(s): Avapritinib,Inapplicable
Therapeutic Area: Hematology Brand Name: Ayvakit
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Sanofi
Deal Size: $9,500.0 million Upfront Cash: $9,100.0 million
Deal Type: Acquisition July 18, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Avapritinib,Inapplicable
Therapeutic Area : Hematology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Sanofi
Deal Size : $9,500.0 million
Deal Type : Acquisition
Sanofi Completes Acquisition of Blueprint Medicines
Details : Through the acquisition of Avapritinib, a small molecule targeting KIT/PDGFA receptor, the deal aims to advance precision medicine in oncology.
Product Name : Ayvakit
Product Type : Miscellaneous
Upfront Cash : $9,100.0 million
July 18, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The acquisition includes a rare immunology disease medicine, Ayvakit/Ayvakyt (avapritinib), approved in the US and the EU, and a promising advanced and early-stage immunology pipeline.
Lead Product(s): Avapritinib,Inapplicable
Therapeutic Area: Oncology Brand Name: Ayvakit
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Sanofi
Deal Size: $9,500.0 million Upfront Cash: $9,100.0 million
Deal Type: Acquisition June 02, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Avapritinib,Inapplicable
Therapeutic Area : Oncology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Sanofi
Deal Size : $9,500.0 million
Deal Type : Acquisition
Sanofi to buy US biopharma group Blueprint for up to $9.5 billion
Details : The acquisition includes a rare immunology disease medicine, Ayvakit/Ayvakyt (avapritinib), approved in the US and the EU, and a promising advanced and early-stage immunology pipeline.
Product Name : Ayvakit
Product Type : Miscellaneous
Upfront Cash : $9,100.0 million
June 02, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Avapritinib is a Other Small Molecule drug candidate, which is currently being evaluated in phase IV clinical studies for the treatment of Mastocytosis, Systemic.
Lead Product(s): Avapritinib,Inapplicable
Therapeutic Area: Hematology Brand Name: Undisclosed
Study Phase: Phase IVProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 24, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Avapritinib,Inapplicable
Therapeutic Area : Hematology
Highest Development Status : Phase IV
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Avapritinib is a Other Small Molecule drug candidate, which is currently being evaluated in phase IV clinical studies for the treatment of Mastocytosis, Systemic.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
December 24, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Ayvakit (avapritinib) is a tyrosine kinase inhibitor that targets KIT D816V, PDGFRA and PDGFRA D842 mutants, indicated for gastrointestinal stromal tumors.
Lead Product(s): Avapritinib,Inapplicable
Therapeutic Area: Oncology Brand Name: Ayvakit
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Blueprint Medicines
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable August 15, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Avapritinib,Inapplicable
Therapeutic Area : Oncology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Blueprint Medicines
Deal Size : Inapplicable
Deal Type : Inapplicable
CStone gets NMPA Nod for Local Manufacturing of AYVAKIT Tablets in China
Details : Ayvakit (avapritinib) is a tyrosine kinase inhibitor that targets KIT D816V, PDGFRA and PDGFRA D842 mutants, indicated for gastrointestinal stromal tumors.
Product Name : Ayvakit
Product Type : Miscellaneous
Upfront Cash : Inapplicable
August 15, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Under the agreement, CStone grants promotion rights of precision therapy ayvakit (avapritinib tablets) in mainland China to Hengrui.
Lead Product(s): Avapritinib,Inapplicable
Therapeutic Area: Oncology Brand Name: Ayvakit
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Jiangsu Hengrui Medicine
Deal Size: Undisclosed Upfront Cash: $4.8 million
Deal Type: Agreement July 03, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Avapritinib,Inapplicable
Therapeutic Area : Oncology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Jiangsu Hengrui Medicine
Deal Size : Undisclosed
Deal Type : Agreement
CStone and Hengrui Enter into Promotion Agreement of AYVAKIT in Mainland China
Details : Under the agreement, CStone grants promotion rights of precision therapy ayvakit (avapritinib tablets) in mainland China to Hengrui.
Product Name : Ayvakit
Product Type : Miscellaneous
Upfront Cash : $4.8 million
July 03, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Ayvakit (avapritinib) is a tyrosine kinase inhibitor that targets KIT D816V, PDGFRA and PDGFRA D842 mutants, indicated for gastrointestinal stromal tumors.
Lead Product(s): Avapritinib,Inapplicable
Therapeutic Area: Oncology Brand Name: Ayvakit
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Blueprint Medicines
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable June 13, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Avapritinib,Inapplicable
Therapeutic Area : Oncology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Blueprint Medicines
Deal Size : Inapplicable
Deal Type : Inapplicable
CStone Secures China NMPA Approval for AYVAKIT Manufacturing Localization
Details : Ayvakit (avapritinib) is a tyrosine kinase inhibitor that targets KIT D816V, PDGFRA and PDGFRA D842 mutants, indicated for gastrointestinal stromal tumors.
Product Name : Ayvakit
Product Type : Miscellaneous
Upfront Cash : Inapplicable
June 13, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Avapritinib is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Mastocytosis, Systemic-associated with Hematologic Neoplasms.
Lead Product(s): Avapritinib,Decitabine,Cedazuridine
Therapeutic Area: Oncology Brand Name: Undisclosed
Study Phase: Phase IProduct Type: Miscellaneous
Sponsor: Blueprint Medicines
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable March 25, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Avapritinib,Decitabine,Cedazuridine
Therapeutic Area : Oncology
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Blueprint Medicines
Deal Size : Inapplicable
Deal Type : Inapplicable
Avapritinib With Decitabine in Patients With SM-AHN
Details : Avapritinib is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Mastocytosis, Systemic-associated with Hematologic Neoplasms.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
March 25, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Ayvakyt (avapritinib) is a kinase inhibitor, works by potently and selectively target KIT D816V. It is approved by european commission for the treatment of indolent systemic mastocytosis (ISM).
Lead Product(s): Avapritinib,Inapplicable
Therapeutic Area: Hematology Brand Name: Ayvakyt
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 13, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Avapritinib,Inapplicable
Therapeutic Area : Hematology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
EC Approves Treatment for Rare Haematological Disorder
Details : Ayvakyt (avapritinib) is a kinase inhibitor, works by potently and selectively target KIT D816V. It is approved by european commission for the treatment of indolent systemic mastocytosis (ISM).
Product Name : Ayvakyt
Product Type : Miscellaneous
Upfront Cash : Inapplicable
December 13, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
AYVAKYT® (avapritinib) is a kinase inhibitor approved by the European Commission for the treatment of adult patients with aggressive systemic mastocytosis (ASM), systemic mastocytosis with an associated hematological neoplasm (SM-AHN) or mast cell leukemia (MCL).
Lead Product(s): Avapritinib,Inapplicable
Therapeutic Area: Hematology Brand Name: Ayvakit
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable November 10, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Avapritinib,Inapplicable
Therapeutic Area : Hematology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Blueprint Medicines' AYVAKYT® (avapritinib) Receives Positive CHMP Opinion as the First and Only ...
Details : AYVAKYT® (avapritinib) is a kinase inhibitor approved by the European Commission for the treatment of adult patients with aggressive systemic mastocytosis (ASM), systemic mastocytosis with an associated hematological neoplasm (SM-AHN) or mast cell leuke...
Product Name : Ayvakit
Product Type : Miscellaneous
Upfront Cash : Inapplicable
November 10, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Ayvakit (avapritinib) is a tyrosine kinase inhibitor that targets KIT D816V, PDGFRA and PDGFRA D842 mutants, indicated for gastrointestinal stromal tumors.
Lead Product(s): Avapritinib,Inapplicable
Therapeutic Area: Oncology Brand Name: Ayvakit
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Genetron Health
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 27, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Avapritinib,Inapplicable
Therapeutic Area : Oncology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Genetron Health
Deal Size : Inapplicable
Deal Type : Inapplicable
CStone gets NMPA Approval for AYVAKIT CDx Kit Developed with Genetron
Details : Ayvakit (avapritinib) is a tyrosine kinase inhibitor that targets KIT D816V, PDGFRA and PDGFRA D842 mutants, indicated for gastrointestinal stromal tumors.
Product Name : Ayvakit
Product Type : Miscellaneous
Upfront Cash : Inapplicable
September 27, 2023

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : AYVAKIT
Dosage Form : TABLET;ORAL
Dosage Strength : 100MG
Packaging :
Approval Date : 2020-01-09
Application Number : 212608
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : AYVAKIT
Dosage Form : TABLET;ORAL
Dosage Strength : 200MG
Packaging :
Approval Date : 2020-01-09
Application Number : 212608
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : AYVAKIT
Dosage Form : TABLET;ORAL
Dosage Strength : 300MG
Packaging :
Approval Date : 2020-01-09
Application Number : 212608
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : AYVAKIT
Dosage Form : TABLET;ORAL
Dosage Strength : 25MG
Packaging :
Approval Date : 2021-06-16
Application Number : 212608
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : AYVAKIT
Dosage Form : TABLET;ORAL
Dosage Strength : 50MG
Packaging :
Approval Date : 2021-06-16
Application Number : 212608
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Approved
Registration Country : Sweden
Brand Name : Ayvakyt
Dosage Form : Film Coated Tablet
Dosage Strength : 100mg
Packaging :
Approval Date : 24/09/2020
Application Number : 20190701000318
Regulatory Info : Approved
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Approved
Registration Country : Sweden
Brand Name : Ayvakyt
Dosage Form : Film Coated Tablet
Dosage Strength : 200mg
Packaging :
Approval Date : 24/09/2020
Application Number : 20190701000325
Regulatory Info : Approved
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Approved
Registration Country : Sweden
Brand Name : Ayvakyt
Dosage Form : Film Coated Tablet
Dosage Strength : 25mg
Packaging :
Approval Date : 24/03/2022
Application Number : 20210205000024
Regulatory Info : Approved
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Approved
Registration Country : Sweden
Brand Name : Ayvakyt
Dosage Form : Film Coated Tablet
Dosage Strength : 300mg
Packaging :
Approval Date : 24/09/2020
Application Number : 20190701000332
Regulatory Info : Approved
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Approved
Registration Country : Sweden
Brand Name : Ayvakyt
Dosage Form : Film Coated Tablet
Dosage Strength : 50mg
Packaging :
Approval Date : 24/03/2022
Application Number : 20210205000031
Regulatory Info : Approved
Registration Country : Sweden

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : AYVAKIT
Dosage Form : TABLET;ORAL
Dosage Strength : 100MG
Approval Date : 2020-01-09
Application Number : 212608
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : AYVAKIT
Dosage Form : TABLET;ORAL
Dosage Strength : 200MG
Approval Date : 2020-01-09
Application Number : 212608
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : AYVAKIT
Dosage Form : TABLET;ORAL
Dosage Strength : 300MG
Approval Date : 2020-01-09
Application Number : 212608
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : AYVAKIT
Dosage Form : TABLET;ORAL
Dosage Strength : 25MG
Approval Date : 2021-06-16
Application Number : 212608
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : AYVAKIT
Dosage Form : TABLET;ORAL
Dosage Strength : 50MG
Approval Date : 2021-06-16
Application Number : 212608
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Approved
Registration Country : Sweden
Brand Name : Ayvakyt
Dosage Form : Film Coated Tablet
Dosage Strength : 100mg
Packaging :
Approval Date : 24/09/2020
Application Number : 20190701000318
Regulatory Info : Approved
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Approved
Registration Country : Sweden
Brand Name : Ayvakyt
Dosage Form : Film Coated Tablet
Dosage Strength : 25mg
Packaging :
Approval Date : 24/03/2022
Application Number : 20210205000024
Regulatory Info : Approved
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Allowed
Registration Country : Switzerland
Brand Name : Ayvakyt
Dosage Form : Film Coated Tablet
Dosage Strength : 25mg
Packaging :
Approval Date : 06/07/2023
Application Number : 68294
Regulatory Info : Allowed
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Allowed
Registration Country : Switzerland
Brand Name : Ayvakyt
Dosage Form : Film Coated Tablet
Dosage Strength : 200mg
Packaging :
Approval Date : 06/07/2023
Application Number : 68294
Regulatory Info : Allowed
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Estonia
Brand Name : Ayvakyt
Dosage Form : Film-Coated Tablet
Dosage Strength : 100mg
Packaging :
Approval Date :
Application Number :
Regulatory Info : Prescription
Registration Country : Estonia

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Estonia
Brand Name : Ayvakyt
Dosage Form : Film-Coated Tablet
Dosage Strength : 300mg
Packaging :
Approval Date :
Application Number :
Regulatory Info : Prescription
Registration Country : Estonia

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Authorized
Registration Country : Spain
Brand Name : Ayvakyt
Dosage Form : Film Coated Tablet
Dosage Strength : 25MG
Packaging :
Approval Date : 26-05-2022
Application Number : 1201473004
Regulatory Info : Authorized
Registration Country : Spain

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Authorized
Registration Country : Spain
Brand Name : Ayvakyt
Dosage Form : Film Coated Tablet
Dosage Strength : 100MG
Packaging :
Approval Date : 26-05-2022
Application Number : 1201473001
Regulatory Info : Authorized
Registration Country : Spain

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Denmark
Brand Name : Ayvakyt
Dosage Form : Film Coated Tablet
Dosage Strength : 100mg
Packaging :
Approval Date : 25-09-2020
Application Number : 28106301219
Regulatory Info : Prescription
Registration Country : Denmark

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Denmark
Brand Name : Ayvakyt
Dosage Form : Film Coated Tablet
Dosage Strength : 200mg
Packaging :
Approval Date : 25-09-2020
Application Number : 28106301319
Regulatory Info : Prescription
Registration Country : Denmark

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]https://www.pharmacompass.com/radio-compass-blog/fda-okays-50-new-drugs-in-2024-bms-cobenfy-lilly-s-kisunla-lead-pack-of-breakthrough-therapies
Global Sales Information

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
10
PharmaCompass offers a list of Avapritinib API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Avapritinib manufacturer or Avapritinib supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Avapritinib manufacturer or Avapritinib supplier.
PharmaCompass also assists you with knowing the Avapritinib API Price utilized in the formulation of products. Avapritinib API Price is not always fixed or binding as the Avapritinib Price is obtained through a variety of data sources. The Avapritinib Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A BLU-285 (Avapritinib) manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of BLU-285 (Avapritinib), including repackagers and relabelers. The FDA regulates BLU-285 (Avapritinib) manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. BLU-285 (Avapritinib) API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of BLU-285 (Avapritinib) manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A BLU-285 (Avapritinib) supplier is an individual or a company that provides BLU-285 (Avapritinib) active pharmaceutical ingredient (API) or BLU-285 (Avapritinib) finished formulations upon request. The BLU-285 (Avapritinib) suppliers may include BLU-285 (Avapritinib) API manufacturers, exporters, distributors and traders.
click here to find a list of BLU-285 (Avapritinib) suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing BLU-285 (Avapritinib) as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for BLU-285 (Avapritinib) API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture BLU-285 (Avapritinib) as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain BLU-285 (Avapritinib) and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a BLU-285 (Avapritinib) NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of BLU-285 (Avapritinib) suppliers with NDC on PharmaCompass.
BLU-285 (Avapritinib) Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of BLU-285 (Avapritinib) GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right BLU-285 (Avapritinib) GMP manufacturer or BLU-285 (Avapritinib) GMP API supplier for your needs.
A BLU-285 (Avapritinib) CoA (Certificate of Analysis) is a formal document that attests to BLU-285 (Avapritinib)'s compliance with BLU-285 (Avapritinib) specifications and serves as a tool for batch-level quality control.
BLU-285 (Avapritinib) CoA mostly includes findings from lab analyses of a specific batch. For each BLU-285 (Avapritinib) CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
BLU-285 (Avapritinib) may be tested according to a variety of international standards, such as European Pharmacopoeia (BLU-285 (Avapritinib) EP), BLU-285 (Avapritinib) JP (Japanese Pharmacopeia) and the US Pharmacopoeia (BLU-285 (Avapritinib) USP).

