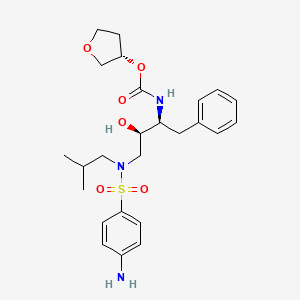

1. (3s-(3r*(1r*,2s*)))-(3-(((4-aminophenyl)sulfonyl)(2-methylpropyl)amino)-2-hydroxy-1-(phenylmethyl)propyl) Tetrahydro-3-furanyl Carbamate
2. Agenerase
3. Tetrahydro-3-furyl N-(3-(4-amino-n-isobutylbenzenesulfonamido)-1-benzyl-2-hydroxypropyl)carbamate
4. Vertex Vx478
5. Vx 478
6. Vx-478
1. 161814-49-9
2. Agenerase
3. Prozei
4. Vx-478
5. 141w94
6. Kvx-478
7. Amprenavir (agenerase)
8. Vx 478
9. Chebi:40050
10. (3s)-tetrahydro-3-furanyl ((1s,2r)-3-(((4-aminophenyl)sulfonyl)(2-methylpropyl)amino)-2-hydroxy-1-(phenylmethyl)propyl)carbamate
11. 5s0w860xnr
12. (3s-(3r*(1r*,2s*)))-(3-(((4-aminophenyl)sulfonyl)(2-methylpropyl)amino)-2-hydroxy-1-(phenylmethyl)propyl) Tetrahydro-3-furanyl Carbamate
13. J05ae05
14. Vertex Vx478
15. 141 W94
16. Amv
17. Carbamic Acid, ((1s,2r)-3-(((4-aminophenyl)sulfonyl)(2-methylpropyl)amino)-2-hydroxy-1-(phenylmethyl)propyl)-, (3s)-tetrahydro-3-furanyl Ester
18. Amprenavir [usan]
19. (3s)-oxolan-3-yl N-[(2s,3r)-3-hydroxy-4-[n-(2-methylpropyl)(4-aminobenzene)sulfonamido]-1-phenylbutan-2-yl]carbamate
20. [(3s)-oxolan-3-yl] N-[(2s,3r)-4-[(4-aminophenyl)sulfonyl-(2-methylpropyl)amino]-3-hydroxy-1-phenylbutan-2-yl]carbamate
21. {3-[(4-amino-benzenesulfonyl)-isobutyl-amino]-1-benzyl-2-hydroxy-propyl}-carbamic Acid Tetrahydro-furan-3-yl Ester
22. Drg-0258
23. Agenerase (tm)
24. Agenerase (tn)
25. (s)-tetrahydrofuran-3-yl (2s,3r)-4-(4-amino-n-isobutylphenylsulfonamido)-3-hydroxy-1-phenylbutan-2-ylcarbamate
26. [(3s)-tetrahydrofuran-3-yl] N-[(1s,2r)-3-[(4-aminophenyl)sulfonyl-isobutyl-amino]-1-benzyl-2-hydroxy-propyl]carbamate
27. Gna & Amprenavir
28. Hha & Amprenavir
29. Hsdb 7157
30. Unii-5s0w860xnr
31. Amprenavir (jan/usan/inn)
32. Amprenavir [usan:inn:ban]
33. Tetrahydro-3-furyl N-(3-(4-amino-n-isobutylbenzenesulfonamido)-1-benzyl-2-hydroxypropyl)carbamate
34. 1hpv
35. 3ekp
36. 3ekv
37. 3nuj
38. 3nuo
39. Vx478
40. Ncgc00159461-02
41. 1t7j
42. 3nu3
43. 3nu4
44. 3nu5
45. 3nu6
46. 3nu9
47. 3sm2
48. Amprenavir [mi]
49. Amprenavir [inn]
50. Amprenavir [jan]
51. Amprenavir [hsdb]
52. Amprenavir [vandf]
53. Apv & Aag
54. Apv & Hsa
55. Chembl116
56. Amprenavir [mart.]
57. Amprenavir [who-dd]
58. Schembl34151
59. (3s)-tetrahydro-3-furyl ((alphas)-alpha-((1r-1-hydroxy-2-(n(sup 1)-isobutylsulfanilamido)ethyl)phenethyl)carbamate
60. Mls006011492
61. Amprenavir [ema Epar]
62. Bidd:gt0398
63. Amprenavir [orange Book]
64. Dtxsid5046061
65. Amprenavir & Human Serum Albumin
66. 3s43
67. 3s45
68. Hms2090n10
69. Zinc3809192
70. Bdbm50215393
71. Mfcd00934214
72. S1639
73. Amprenavir & Alpha1-acid Glycoprotein
74. Akos000280844
75. Am84544
76. Bcp9000297
77. Ccg-269742
78. Cs-1410
79. Db00701
80. Ncgc00159461-07
81. Ncgc00159461-08
82. (3s)-oxolan-3-yl N-[(2s,3r)-3-hydroxy-4-[n-(2-methylpropyl)4-aminobenzenesulfonamido]-1-phenylbutan-2-yl]carbamate
83. 4-amino-n-((2 Syn,3s)-2-hydroxy-4-phenyl-3-((s)-tetrahydrofuran-3-yloxycarbonylamino)-butyl)-n-isobutyl-benzenesulfonamide
84. Amprenavir 100 Microg/ml In Acetonitrile
85. As-30915
86. Carbamic Acid, ((1s,2r)-3-(((4-aminophenyl)sulfonyl)(2-methylpropyl)amino)-2-hydroxy-1-(phenylmethyl)propyl)-, (3s)-tetrahydro-3-furanyl Ester & Galanthus Nivalis Agglutinin (gna)
87. Carbamic Acid, (3-(((4-aminophenyl)sulfonyl)(2-methylpropyl)amino)-2-hydroxy-1-(phenylmethyl)propyl)-, Tetrahydro-3-furanyl Ester, (3s-(3r*(1s*,2r*)))-
88. Carbamic Acid, N-[(1s,2r)-3-[[(4-aminophenyl)sulfonyl](2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]-, (3s)-tetrahydro-3-furanyl Ester
89. Hy-17430
90. Smr003885056
91. Bcp0726000051
92. Sw219687-1
93. C08086
94. D00894
95. Ab01275534-01
96. Ab01275534_02
97. 814a499
98. A810295
99. Q422198
100. Sr-05000001530
101. Sr-05000001530-1
102. Z1557399789
103. (3s)-tetrahydro-3-furyl ((.alpha.s)-.alpha.-((1r-1-hydroxy-2-(n1-isobutylsulfanilamido)ethyl)phenethyl)carbamate
104. (3s)-tetrahydrofuran-3-yl [(1s,2r)-3-{[(4-aminophenyl)sulfonyl](2-methylpropyl)amino}-1-benzyl-2-hydroxypropyl]carbamate
105. (3s)-tetrahydrofuran-3-yl [(2s,3r)-4-{[(4-aminophenyl)sulfonyl](2-methylpropyl)amino}-3-hydroxy-1-phenylbutan-2-yl]carbamate
106. [(3s)-oxolan-3-yl] N-[(2s,3r)-4-[(4-aminophenyl)sulfonyl-(2-methylpropyl)amino]-3-oxidanyl-1-phenyl-butan-2-yl]carbamate
107. Carbamic Acid, ((1s,2r)-3-(((4-aminophenyl)sulfonyl)(2-methylpropyl)amino)-2-hydroxy-1-(phenylmethyl)propyl)-, (3s)-tetrahydro-3-furanyl Ester & Hippeastrum Hybrid Agglutinin( Hha)
108. Carbamic Acid,[(1s,2r)-3-[[(4-aminophenyl)sulfonyl](2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]-,(3s)-tetrahydro-3-furanyl Ester
109. N-[(1s,2r)-3-[[(4-aminophenyl)sulfonyl](2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic Acid (3s)-tetrahydro-3-furanyl Ester
110. N-[(2s,3r)-4-[(4-aminophenyl)sulfonyl-(2-methylpropyl)amino]-3-hydroxy-1-phenylbutan-2-yl]carbamic Acid [(3s)-3-oxolanyl] Ester
| Molecular Weight | 505.6 g/mol |
|---|---|
| Molecular Formula | C25H35N3O6S |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 12 |
| Exact Mass | 505.22465702 g/mol |
| Monoisotopic Mass | 505.22465702 g/mol |
| Topological Polar Surface Area | 140 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 745 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Amprenavir is indicated in combination with other antiretroviral agents in the treatment of HIV-1 infection. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 118
Amprenavir is a human immunodeficiency virus (HIV)-protease inhibitor. The use of amprenavir for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in combination with other antiretrovirals is based on analyses of plasma HIV-RNA levels and CD4 cell counts in controlled studies of up to 24 weeks duration. Results from controlled trials evaluating the long-term suppression of HIV-RNA or disease progression with amprenavir have not yet been obtained.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 118
Amprenavir is a viral protease inhibitor with specificity for the HIV protease enzyme. The resistance profile of amprenavir appears to differ from that of other protease inhibitors such as saquinavir and indinavir. Twelve hours after single-dose administration of amprenavir 1200 mg to HIV-infected individuals, the mean plasma concentration of the drug was more than 10-fold greater than the 50% inhibitory concentration for HIV-1IIIB in peripheral blood lymphocytes. In a small nonblind study, amprenavir monotherapy increased CD4+ cell count and decreased viral load in 37 patients with HIV infection and no previous exposure to protease inhibitor therapy. Combination therapy comprising amprenavir and other antiretroviral agents (abacavir, zidovudine, lamivudine, indinavir, saquinavir or nelfinavir) decreased viral load and increased CD4+ cell counts in patients with HIV infection. Antiviral efficacy was maintained during up to 24 weeks' follow-up.
PMID:9617598 Adkins JC, Faulds D; Drugs 55 (6): 837-42 (1998)
The usually recommended dosage of amprenavir oral solution (22.5 mg/kg twice daily) provides a propylene glycol intake of 1650 mg/kg daily; however, an acceptable intake of propylene glycol used as an excipient in pharmaceuticals has not been established to date. Propylene glycol is metabolized in the liver by the alcohol and aldehyde dehydrogenase enzyme pathway, and the possibility exists that young infants, patients with renal or hepatic impairment, and certain patient groups (females, Asians, Native Alaskans, Native Americans) may be at increased risk of propylene glycol-associated adverse effects if they receive amprenavir oral solution because of diminished ability to metabolize propylene glycol. Therefore, amprenavir oral solution is contraindicated during pregnancy; in infants younger than 4 years of age; in patients with renal or hepatic failure; and in patients receiving disulfiram or metronidazole. In addition, although metabolism of propylene glycol has not been specifically studied in these patient groups, the possibility that females may have lower concentrations of alcohol dehydrogenase compared with males and that certain ethnic populations (Asians, Native Alaskans, Native Americans) may have alcohol dehydrogenase polymorphism should be considered.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 626
Because amprenavir oral solution contains large amounts of propylene glycol and because young infants may be at increased risk of propylene glycol-associated adverse effects, the oral solution is contraindicated in pediatric patients younger than 4 years of age. Propylene glycol is metabolized in the liver by the alcohol and aldehyde dehydrogenase enzyme pathway. Although alcohol dehydrogenase is present in human fetal liver at 2 months of gestational age, this represent only about 3% of the activity reported in adults. Limited data indicate that alcohol dehydrogenase activity in infants 12-30 months of age is equal to or greater than that reported in adults. Oral or IV administration of various drugs (e.g., multivitamins) containing high concentrations of propylene glycol in pediatric patients has resulted in various propylene glycol-associated adverse effects, including hyperosmolality, lactic acidosis, respiratory depression, and seizures.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 627
Patients being treated with amprenavir oral solution should be closely monitored for propylene glycol associated side effects including hemolysis, hyperosmolality, lactic acidosis, renal toxicity, seizures, stupor, and tachycardia.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 121
The pharmacokinetics of amprenavir do not differ between females and males or between Blacks and non-Blacks. However, amprenavir oral solution contains a large amount of propylene glycol and because Asians, Eskimos, Native Americans, and women have a decreased ability to metabolize this compound, they may have an increased risk of developing propylene glycol-associated side effects.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 119
For more Drug Warnings (Complete) data for AMPRENAVIR (18 total), please visit the HSDB record page.
For the treatment of HIV-1 infection in combination with other antiretroviral agents.
FDA Label
Agenerase, in combination with other antiretroviral agents, is indicated for the treatment of protease inhibitor (PI) experienced HIV-1 infected adults and children above the age of 4 years. Agenerase capsules should normally be administered with low dose ritonavir as a pharmacokinetic enhancer of amprenavir (see sections 4. 2 and 4. 5). The choice of amprenavir should be based on individual viral resistance testing and treatment history of patients (see section 5. 1).
The benefit of Agenerase boosted with ritonavir has not been demonstrated in PI nave patients (see section 5. 1)
Amprenavir is a protease inhibitor with activity against Human Immunodeficiency Virus Type 1 (HIV-1). Protease inhibitors block the part of HIV called protease. HIV-1 protease is an enzyme required for the proteolytic cleavage of the viral polyprotein precursors into the individual functional proteins found in infectious HIV-1. Amprenavir binds to the protease active site and inhibits the activity of the enzyme. This inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature non-infectious viral particles. Protease inhibitors are almost always used in combination with at least two other anti-HIV drugs.
Antibiotics, Antitubercular
Substances obtained from various species of microorganisms that are, alone or in combination with other agents, of use in treating various forms of tuberculosis; most of these agents are merely bacteriostatic, induce resistance in the organisms, and may be toxic. (See all compounds classified as Antibiotics, Antitubercular.)
HIV Protease Inhibitors
Inhibitors of HIV PROTEASE, an enzyme required for production of proteins needed for viral assembly. (See all compounds classified as HIV Protease Inhibitors.)
Anti-HIV Agents
Agents used to treat AIDS and/or stop the spread of the HIV infection. These do not include drugs used to treat symptoms or opportunistic infections associated with AIDS. (See all compounds classified as Anti-HIV Agents.)
J05AE05
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AE - Protease inhibitors
J05AE05 - Amprenavir
Absorption
Rapidly absorbed after oral administration in HIV-1-infected patients with a time to peak concentration (Tmax) typically between 1 and 2 hours after a single oral dose. The absolute oral bioavailability of amprenavir in humans has not been established.
Amprenavir is absorbed rapidly after oral administration. Taking amprenavir with a standard meal reduces the plasma AUC by only about 13%, but high-fat meals may have greater effects and should be avoided.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1371
Only minimal amounts of amprenavir are eliminated unchanged in urine or feces; less than 3% of a dose is eliminated unchanged in urine. Following a single oral dose of radiolabeled amprenavir, approximately 14% of the dose is eliminated in urine and 75% is eliminated in feces; 2 metabolites account for more than 90% of radioactivity in feces.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 632
Distribution of amprenavir into body tissues and fluids has not been fully characterized. Studies in rats indicate that amprenavir is distributed to a variety of tissues following oral administration. The apparent volume of distribution of amprenavir in healthy adults is approximately 430 L.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 631
It is not known whether amprenavir crosses the human placenta; however, the drug crosses the placenta in rats. Information from an ex vivo human placental model for transplacental passage indicates that amprenavir crosses the human placenta. Although it is not known whether amprenavir is distributed in human milk, the drug is distributed into milk in rats.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 631
In patients with hepatic impairment, the peak plasma concentration and AUC of amprenavir may be increased. In adults with moderate cirrhosis who received a single 600-mg oral dose of amprenavir given as liquid-filled capsules, the AUC (0-4 hours) of the drug averaged 25.76 ug hour/mL compared with 12 ug hour/ml in healthy adults. In adults with severe cirrhosis who received the same dose, peak plasma concentrations averaged 9.43 ug/ml and the AUC (0-4 hours) averaged 38.66 ug hour/ml compared with 4.9 ug/ml or 12 ug hour/ml, respectively, in healthy adults.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 631
Hepatic. Amprenavir is metabolized in the liver by the cytochrome P450 3A4 (CYP3A4) enzyme system. The 2 major metabolites result from oxidation of the tetrahydrofuran and aniline moieties. Glucuronide conjugates of oxidized metabolites have been identified as minor metabolites in urine and feces.
The metabolic fate of amprenavir has not been fully determined, but the drug is metabolized in the liver. Amprenavir is metabolized principally by the cytochrome P450 (CYP) isoenzyme 3A4. The 2 major metabolites of the drug result from oxidation of the tetrahydrofuran and aniline moieties; glucuronide conjugates of oxidized metabolites have been identified as minor metabolites in urine and feces.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 632
7.1-10.6 hours
The plasma elimination half-life of amprenavir in HIV-infected adults with normal renal and hepatic function ranges from 7.1-10.6 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 632
Amprenavir inhibits the HIV viral proteinase enzyme which prevents cleavage of the gag-pol polyprotein, resulting in noninfectious, immature viral particles.
Amprenavir acts by reversibly binding to the active site of HIV protease. This prevents polypeptide processing and subsequent viral maturation.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1371
Although the complete mechanism(s) of antiviral activity of amprenavir has not been fully elucidated, amprenavir apparently inhibits replication of human immunodeficiency virus type 1 (HIV-1) and type 2 (HIV-2) by interfering with HIV protease. The drug, therefore, exerts a virustatic effect against retroviruses by acting as an HIV protease inhibitor.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 630
Amprenavir is a selective, competitive, reversible inhibitor of HIV protease. HIV protease, an aspartic endopeptidase that functions as a homodimer, plays an essential role in the HIV replication cycle and the formation of infectious virus. During HIV replication, HIV protease cleaves viral polypeptide products of the gag and gag-pol genes (i.e., p55 and p160) to form structural proteins of the virion core (i.e., p17, p24, p9, and p7) and essential viral enzymes (i.e., reverse transcriptase, integrase, protease). By interfering with the formation of these essential proteins and enzymes, amprenavir blocks maturation of the virus and causes the formation of nonfunctional, immature, noninfectious virions. Amprenavir is active in both acutely and chronically infected cells since it targets the HIV replication cycle after translation and before assembly. Thus, the drug is active in a subset of chronically infected cells (e.g., monocytes, macrophages) that generally are not affected by nucleoside reverse transcriptase inhibitors (e.g., abacavir, didanosine, lamivudine, stavudine, zalcitabine, zidovudine). Amprenavir does not affect early stages of the HIV replication cycle; however, the drug interferes with the production of infectious HIV and limits further infectious spread of the virus.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 630
Unlike nucleoside reverse transcriptase inhibitors, the antiretroviral activity of amprenavir does not depend on intracellular conversion to an active metabolite. Amprenavir and other HIV protease inhibitors (e.g., indinavir, lopinavir, nelfinavir, ritonavir, saquinavir) act at a different stage of the HIV replication cycle than other currently available antiretroviral agents, including nucleoside reverse transcriptase inhibitors and nonnucleoside reverse transcriptase inhibitors.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 630
Amprenavir is a highly specific inhibitor of HIV protease. Amprenavir has low affinity for human aspartic endopeptidases such as pepsin, renin, gastricin, cathepsin D, and cathepsin E. Results of in vitro studies using MT-4 cells indicate that amprenavir is not cytotoxic at concentrations up to 100 um.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 630