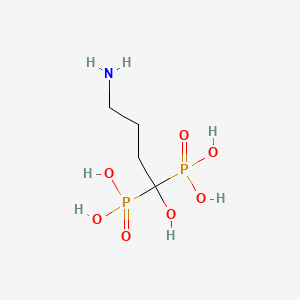

1. 4-amino-1-hydroxybutylidene 1,1-biphosphonate
2. Alendronate
3. Alendronate Monosodium Salt, Trihydrate
4. Alendronate Sodium
5. Aminohydroxybutane Bisphosphonate
6. Fosamax
7. Mk 217
8. Mk-217
9. Mk217
1. 66376-36-1
2. Acido Alendronico
3. Acide Alendronique
4. Acidum Alendronicum
5. (4-amino-1-hydroxybutylidene)bisphosphonic Acid
6. Abdp
7. Acide Alendronique [inn-french]
8. Acido Alendronico [inn-spanish]
9. Acidum Alendronicum [inn-latin]
10. (4-amino-1-hydroxybutylidene)diphosphonic Acid
11. 4-amino-1-hydroxybutane-1,1-diphosphonic Acid
12. Phosphonic Acid, (4-amino-1-hydroxybutylidene)bis-
13. (4-amino-1-hydroxybutane-1,1-diyl)bis(phosphonic Acid)
14. Unii-x1j18r4w8p
15. Chebi:2567
16. X1j18r4w8p
17. Hsdb 7990
18. 4-amino-1-hydroxybutylidene-1,1-bis(phosphonic Acid)
19. Dtxsid5022568
20. P,p'-(4-amino-1-hydroxybutylidene)bisphosphonic Acid
21. Alendronate (mart.)
22. Alendronate [mart.]
23. Acide Alendronique (inn-french)
24. Acido Alendronico (inn-spanish)
25. Acidum Alendronicum (inn-latin)
26. Alpha-hydroxy-delta-aminobutylidenediphosphonic Acid
27. Dtxcid202568
28. M05ba04
29. Alendronate
30. Fosamax
31. (4-amino-1-hydroxy-1-phosphonobutyl)phosphonic Acid
32. (4-amino-1-hydroxybutane-1,1-diyl)diphosphonic Acid
33. Bisphosphonate
34. Arendal
35. Alendronate Acid
36. Mk 217
37. Alendronic Acid (inn)
38. Mk-217
39. Chembl870
40. Alendronate Sodium Hydrate
41. (4-amino-1-hydroxy-1-phosphono-butyl)phosphonic Acid
42. Alendronic Acid [inn]
43. Alendronic Acid [inn:ban]
44. Phosphonic Acid, P,p'-(4-amino-1-hydroxybutylidene)bis-
45. Bisphosphonate, 65
46. Ncgc00096054-03
47. 4-amino-1-hydroxybutylidene-1,1-bisphosphonate
48. Sr-05000001906
49. 1yhm
50. Mfcd00868112
51. Abdp-d6
52. 4-amino-1-hydroxybutane-1,1-diphosphonate
53. Alendronate [vandf]
54. Bph 1
55. Oprea1_422906
56. Schembl18898
57. Alendronic Acid [mi]
58. Phosphonic Acid, (4-amino-1-hydroxybutylidene)bis
59. Bidd:gt0180
60. Spectrum1505166
61. Gtpl3141
62. Bdbm25313
63. 66376-36-1 (unlabeled)
64. Alendronic Acid [who-dd]
65. Alendronic Acid [ema Epar]
66. Hy-b0631
67. Krb43739
68. Hsci1_000337
69. S5536
70. Akos001015793
71. Db00630
72. Fa38407
73. Ncgc00096054-01
74. Ncgc00096054-04
75. As-10963
76. Sbi-0206778.p001
77. A2120
78. Cs-0009566
79. Ns00004332
80. 4-amino-1-hydroxybutylidene-bisphosphonic Acid
81. C07752
82. D07119
83. D88506
84. 4-amino-1- Hydroxybutane-1,1-diphosphonate-d6
85. Ab01274863-01
86. (4-amino-1-hydroxybutylidene)-bisphosphonic Acid
87. A835441
88. Q420057
89. Sr-05000001906-1
90. (4-amino-1-hydroxybutane-1,1-diyl)bisphosphonic Acid
91. Brd-k75527158-323-01-3
92. Brd-k75527158-360-04-9
93. Brd-k75527158-360-05-6
94. Brd-k75527158-360-06-4
95. Z56771118
96. (4-azanyl-1-oxidanyl-1-phosphono-butyl)phosphonic Acid
97. .alpha.-hydroxy-.delta.-aminobutylidenediphosphonic Acid
| Molecular Weight | 249.10 g/mol |
|---|---|
| Molecular Formula | C4H13NO7P2 |
| XLogP3 | -6.5 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | Da |
| Monoisotopic Mass | Da |
| Topological Polar Surface Area | 161 |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 257 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Bone Density Conservation Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Alendronate sodium tablets are indicated for the treatment of osteoporosis, alendronate sodium tablets increases bone mass and reduce the incidence of fractures, including those of the hip and spine (vertebral compression fractures). Osteoporosis may be confirmed by the finding of low bone mass (for example, at least 2 standard deviations below the premenopausal mean) or by the presence or history of osteoporotic fracture. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for ALENDRONATE SODIUM (alendronate sodium) tablet (January 2011). Available from, as of October 7, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7ae56813-7fc4-46d1-97b5-f7a4b755fc6b
Alendronate sodium tablets are indicated for the prevention of osteoporosis, alendronate sodium tablets may be considered in postmenopausal women who are at risk of developing osteoporosis and for whom the desired clinical outcome is to maintain bone mass and to reduce the risk of future fracture. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for ALENDRONATE SODIUM (alendronate sodium) tablet (January 2011). Available from, as of October 7, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7ae56813-7fc4-46d1-97b5-f7a4b755fc6b
Alendronate sodium tablets are indicated for treatment to increase bone mass in men with osteoporosis. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for ALENDRONATE SODIUM (alendronate sodium) tablet (January 2011). Available from, as of October 7, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7ae56813-7fc4-46d1-97b5-f7a4b755fc6b
For more Therapeutic Uses (Complete) data for Alendronic acid (7 total), please visit the HSDB record page.
FDA notified healthcare professionals and patients about its ongoing review of data from published studies to evaluate whether use of oral bisphosphonate drugs is associated with an increased risk of cancer of the esophagus. FDA has not concluded that taking an oral bisphosphonate drug increases the risk of esophageal cancer. There are insufficient data to recommend endoscopic screening of asymptomatic patients. FDA will continue to evaluate all available data supporting the safety and effectiveness of bisphosphonate drugs and will update the public when more information becomes available.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011
Bone loss is particularly rapid in postmenopausal women younger than age 60. Risk factors often associated with the development of postmenopausal osteoporosis include early menopause; moderately low bone mass (for example, at least 1 standard deviation below the mean for healthy young adult women); thin body build; Caucasian or Asian race; and family history of osteoporosis. The presence of such risk factors may be important when considering the use of alendronate sodium tablets for prevention of osteoporosis.
US Natl Inst Health; DailyMed. Current Medication Information for ALENDRONATE SODIUM (alendronate sodium) tablet (January 2011). Available from, as of October 7, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7ae56813-7fc4-46d1-97b5-f7a4b755fc6b
/Alendronate sodium is contraindicated in the presence of/: Abnormalities of the esophagus which delay esophageal emptying such as stricture or achalasia; inability to stand or sit upright for at least 30 minutes; hypersensitivity to any component of this product; hypocalcemia.
US Natl Inst Health; DailyMed. Current Medication Information for ALENDRONATE SODIUM (alendronate sodium) tablet (January 2011). Available from, as of October 7, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7ae56813-7fc4-46d1-97b5-f7a4b755fc6b
Alendronate sodium, like other bisphosphonates, may cause local irritation of the upper gastrointestinal mucosa. Esophageal adverse experiences, such as esophagitis, esophageal ulcers and esophageal erosions, occasionally with bleeding and rarely followed by esophageal stricture or perforation, have been reported in patients receiving treatment with alendronate sodium. In some cases these have been severe and required hospitalization. Physicians should therefore be alert to any signs or symptoms signaling a possible esophageal reaction and patients should be instructed to discontinue alendronate sodium and seek medical attention if they develop dysphagia, odynophagia, retrosternal pain or new or worsening heartburn.
US Natl Inst Health; DailyMed. Current Medication Information for ALENDRONATE SODIUM (alendronate sodium) tablet (January 2011). Available from, as of October 7, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7ae56813-7fc4-46d1-97b5-f7a4b755fc6b
For more Drug Warnings (Complete) data for Alendronic acid (18 total), please visit the HSDB record page.
Alendronic acid is indicated for the treatment and prevention of osteoporosis in men and postmenopausal women, treatment of glucocorticoid-induced osteoporosis, and Paget's disease of bone. However, alendronic acid is not indicated for use in pediatric populations or patients with a creatinine clearance <35mL/min.
FDA Label
FDA-Approved Indications
Bisphosphonates define a class of drugs widely indicated since the 1990s to treat osteoporosis both in men and women. Their effectiveness in treating osteoporosis and other conditions is related to their ability to inhibit bone resorption.
Bone Density Conservation Agents
Agents that inhibit BONE RESORPTION and/or favor BONE MINERALIZATION and BONE REGENERATION. They are used to heal BONE FRACTURES and to treat METABOLIC BONE DISEASES such as OSTEOPOROSIS. (See all compounds classified as Bone Density Conservation Agents.)
M05BA04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
M - Musculo-skeletal system
M05 - Drugs for treatment of bone diseases
M05B - Drugs affecting bone structure and mineralization
M05BA - Bisphosphonates
M05BA04 - Alendronic acid
Absorption
Mean oral bioavailability of alendronic acid in women is 0.64% and in men is 0.59%. Bioavailability of alendronic acid decreases by up to 40% if it is taken within an hour of a meal.
Route of Elimination
Administration of radiolabeled alendronic acid results in 50% recovery in urine within 72 hours. No alendronic acid is recovered in the feces. Men excrete less alendronic acid than women, though race and advanced age do not affect elimination.
Volume of Distribution
28L.
Clearance
71mL/min.
Relative to an intravenous iv reference dose, the mean oral bioavailability of alendronate in women was 0.64% for doses ranging from 5 to 70 mg when administered after an overnight fast and two hours before a standardized breakfast. Oral bioavailability of the 10 mg tablet in men (0.59%) was similar to that in women when administered after an overnight fast and 2 hours before breakfast.
US Natl Inst Health; DailyMed. Current Medication Information for ALENDRONATE SODIUM (alendronate sodium) tablet (January 2011). Available from, as of October 7, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7ae56813-7fc4-46d1-97b5-f7a4b755fc6b
Alendronate sodium 70 mg oral solution and alendronate sodium 70 mg tablet are equally bioavailable.
US Natl Inst Health; DailyMed. Current Medication Information for ALENDRONATE SODIUM (alendronate sodium) tablet (January 2011). Available from, as of October 7, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7ae56813-7fc4-46d1-97b5-f7a4b755fc6b
A study examining the effect of timing of a meal on the bioavailability of alendronate was performed in 49 postmenopausal women. Bioavailability was decreased (by approximately 40%) when 10 mg alendronate was administered either 0.5 or 1 hour before a standardized breakfast, when compared to dosing 2 hours before eating. In studies of treatment and prevention of osteoporosis, alendronate was effective when administered at least 30 minutes before breakfast.
US Natl Inst Health; DailyMed. Current Medication Information for ALENDRONATE SODIUM (alendronate sodium) tablet (January 2011). Available from, as of October 7, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7ae56813-7fc4-46d1-97b5-f7a4b755fc6b
Bioavailability was negligible whether alendronate was administered with or up to two hours after a standardized breakfast.
US Natl Inst Health; DailyMed. Current Medication Information for ALENDRONATE SODIUM (alendronate sodium) tablet (January 2011). Available from, as of October 7, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7ae56813-7fc4-46d1-97b5-f7a4b755fc6b
For more Absorption, Distribution and Excretion (Complete) data for Alendronic acid (10 total), please visit the HSDB record page.
Urinary excretion is the sole method of elimination of alendronic acid and no metabolites are detected upon urine collection.
There is no evidence that alendronate is metabolized in animals or humans.
US Natl Inst Health; DailyMed. Current Medication Information for ALENDRONATE SODIUM (alendronate sodium) tablet (January 2011). Available from, as of October 7, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7ae56813-7fc4-46d1-97b5-f7a4b755fc6b
There is no evidence that alendronate is metabolized in humans or animals. Route of Elimination: Following a single IV dose of [14C]alendronate, approximately 50% of the radioactivity was excreted in the urine within 72 hours and little or no radioactivity was recovered in the feces. Half Life: >10 years
Due to alendronic acid being incorporated into the skeleton, the terminal half life is estimated to be over 10 years.
The terminal half-life in humans is estimated to exceed 10 years, probably reflecting release of alendronate from the skeleton. Based on the above, it is estimated that after 10 years of oral treatment with alendronate sodium (10 mg daily) the amount of alendronate released daily from the skeleton is approximately 25% of that absorbed from the gastrointestinal tract.
US Natl Inst Health; DailyMed. Current Medication Information for ALENDRONATE SODIUM (alendronate sodium) tablet (January 2011). Available from, as of October 7, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7ae56813-7fc4-46d1-97b5-f7a4b755fc6b
Alendronic acid binds to bone hydroxyapatite. Bone resorption causes local acidification, releasing alendronic acid which is that taken into osteoclasts by fluid-phase endocytosis. Endocytic vesicles are acidified, releasing alendronic acid to the cytosol of osteoclasts where they induce apoptosis. Inhibition of osteoclasts results in decreased bone resorption which is shown through decreased urinary calcium, deoxypyridinoline and cross-linked N-telopeptidases of type I collagen.
Animal studies have indicated the following mode of action. At the cellular level, alendronate shows preferential localization to sites of bone resorption, specifically under osteoclasts. The osteoclasts adhere normally to the bone surface but lack the ruffled border that is indicative of active resorption. Alendronate does not interfere with osteoclast recruitment or attachment, but it does inhibit osteoclast activity. Studies in mice on the localization of radioactive (3)H-alendronate in bone showed about 10-fold higher uptake on osteoclast surfaces than on osteoblast surfaces. Bones examined 6 and 49 days after (3)H-alendronate administration in rats and mice, respectively, showed that normal bone was formed on top of the alendronate, which was incorporated inside the matrix. While incorporated in bone matrix, alendronate is not pharmacologically active. Thus, alendronate must be continuously administered to suppress osteoclasts on newly formed resorption surfaces. Histomorphometry in baboons and rats showed that alendronate treatment reduces bone turnover (i.e., the number of sites at which bone is remodeled). In addition, bone formation exceeds bone resorption at these remodeling sites, leading to progressive gains in bone mass.
US Natl Inst Health; DailyMed. Current Medication Information for ALENDRONATE SODIUM (alendronate sodium) tablet (January 2011). Available from, as of October 7, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7ae56813-7fc4-46d1-97b5-f7a4b755fc6b
Alendronate (alendronate sodium hydrate) is a nitrogen-containing bisphosphonate, which combines with the bone surface and reduces osteoclast-mediated bone resorption. It is a third-generation bisphosphonate compound, specifically distributed on the surface of bone resorption and taken into osteoclasts. Under the closed circumstances which is formed with osteoclast and the bone surface, alendronate becomes detached from the bone surface and taken into osteoclast since acid released from osteoclast leads to pH decrease (acidified). The uptaken alendronate blocks the pathway of mevalonic acid synthesis, which is cholesteric synthesis, inhibits the prenylation of GTP binding protein, and decreases the osteoclast's function by influencing the cytoskeleton. This restraint of alendronate in bone resorption against osteoclasts is reversible, showing no cytotoxicity at more than hundredfold concentration level at which action occurs. ...
PMID:12528472 Ohta T et al; Nihon Yakurigaku Zasshi 120 (6): 409-19 (2002)
The differences that exist among individual BPs in terms of mineral binding and biochemical actions may explain differences in their clinical behavior and effectiveness. The classical pharmacological effects of bisphosphonates (BPs) appear to be the result of two key properties: their affinity for bone mineral and their inhibitory effects on osteoclasts. There is new information about both properties. Mineral binding affinities differ among the clinically used BPs and may influence their differential distribution within bone, their biological potency, and their duration of action. The antiresorptive effects of the nitrogen-containing BPs (including alendronate, risedronate, ibandronate, and zoledronate) appear to result from their inhibition of the enzyme farnesyl pyrophosphate synthase (FPPS) in osteoclasts. FPPS is a key enzyme in the mevalonate pathway, which generates isoprenoid lipids utilized for the post-translational modification of small GTP-binding proteins that are essential for osteoclast function. Effects on other cellular targets, such as osteocytes, may also be important. BPs share several common properties as a drug class. However, as with other families of drugs, there are obvious chemical, biochemical, and pharmacological differences among the individual BPs. Each BP has a unique profile that may help to explain potential clinical differences among them, in terms of their speed and duration of action, and effects on fracture reduction.
PMID:18214569 Russell RG et al; Osteoporos Int 19 (6): 733-59 (2008)
BUILDING BLOCK


