


1. 9-((2-hydroxyethoxy)methyl)guanine
2. Aci Sanorania
3. Aci-sanorania
4. Acic
5. Aciclobeta
6. Aciclostad
7. Aciclovir
8. Aciclovir Alonga
9. Aciclovir Sanorania
10. Aciclovir Von Ct
11. Aciclovir-sanorania
12. Acifur
13. Acipen Solutab
14. Acivir
15. Activir
16. Acyclo V
17. Acyclo-v
18. Acycloguanosine
19. Acyclovir
20. Alonga, Aciclovir
21. Antiherpes Creme
22. Avirax
23. Cicloferon
24. Clonorax
25. Cusiviral
26. Genvir
27. Herpetad
28. Herpofug
29. Herpotern
30. Herpoviric
31. Isavir
32. Laciken
33. Mapox
34. Maynar
35. Milavir
36. Opthavir
37. Sodium, Acyclovir
38. Solutab, Acipen
39. Supraviran
40. Viclovir
41. Vipral
42. Virax Puren
43. Virax-puren
44. Viraxpuren
45. Virherpes
46. Virmen
47. Virolex
48. Virupos
49. Virzin
50. Wellcome 248u
51. Wellcome-248u
52. Wellcome248u
53. Zoliparin
54. Zovirax
55. Zyclir
1. Aciclovir Sodium
2. 69657-51-8
3. Sodium Acyclovir
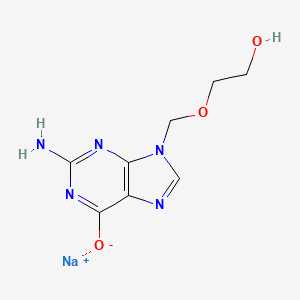
4. Sodium 2-((2-amino-6-oxo-1h-purin-9(6h)-yl)methoxy)ethanolate
5. Acyclovir Sodium Salt
6. Bw 248u Sodium
7. Mfcd01694138
8. Bw-248u Sodium
9. Bw-248u-sodium
10. Sodium 2-amino-9-((2-hydroxyethoxy)methyl)-9h-purin-6-olate
11. Sodium 2-amino-9-[(2-hydroxyethoxy)methyl]purin-6-olate
12. 9-((2-hydroxyethoxy)methyl)guanine Monosodium Salt
13. Aciclovir Natrium
14. Bw 248u
15. Zovirax Sterile Powder
16. 927l42j563
17. Acycloguanosine Sodium (obs.)
18. Unii-927l42j563
19. 6h-purin-6-one, 2-amino-1,9-dihydro-9-((2-hydroxyethoxy)methyl)-, Monosodium Salt
20. Schembl40722
21. Chembl1200380
22. Bcp13172
23. Akos015896105
24. 2-amino-1,9-dihydro-9-((2-hydroxyethoxy)methyl)-6h-purin-6-one Monosodium Salt
25. As-12705
26. Sy027836
27. O12074
28. Q27271485
29. Sodium2-amino-9-((2-hydroxyethoxy)methyl)-9h-purin-6-olate
30. 6h-purin-6-one, 1,9-dihydro-2-amino-9-((2-hydroxyethoxy)methyl)-, Monosodium Salt
| Molecular Weight | 247.19 g/mol |
|---|---|
| Molecular Formula | C8H10N5NaO3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Exact Mass | 247.06813348 g/mol |
| Monoisotopic Mass | 247.06813348 g/mol |
| Topological Polar Surface Area | 122 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 237 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Acyclovir sodium |
| Drug Label | Acyclovir is a synthetic nucleoside analog active against herpes viruses. Acyclovir for Injection USP is a sterile lyophilized powder for intravenous administration only. Each 500 mg vial contains 500 mg of acyclovir and 49 mg of sodium, and each 100... |
| Active Ingredient | Acyclovir sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 50mg base/ml; eq 500mg base/vial; eq 1gm base/vial |
| Market Status | Prescription |
| Company | Bedford; Fresenius Kabi Usa; Aurobindo Pharma |
| 2 of 2 | |
|---|---|
| Drug Name | Acyclovir sodium |
| Drug Label | Acyclovir is a synthetic nucleoside analog active against herpes viruses. Acyclovir for Injection USP is a sterile lyophilized powder for intravenous administration only. Each 500 mg vial contains 500 mg of acyclovir and 49 mg of sodium, and each 100... |
| Active Ingredient | Acyclovir sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 50mg base/ml; eq 500mg base/vial; eq 1gm base/vial |
| Market Status | Prescription |
| Company | Bedford; Fresenius Kabi Usa; Aurobindo Pharma |
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)