
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
Regulatory FDF Prices
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
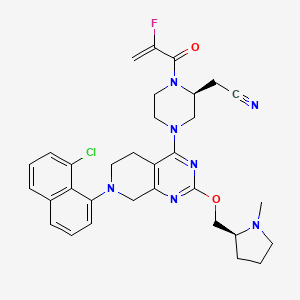

1. 2-((s)-4-(7-(8-chloronaphthalen-1-yl)-2-(((s)-1-methylpyrrolidin-2-yl)methoxy)-5,6,7,8-tetrahydropyrido(3,4-d)pyrimidin-4-yl)-1-(2-fluoroacryloyl)piperazin-2-yl)acetonitrile
2. 2-piperazineacetonitrile, 4-(7-(8-chloro-1-naphthalenyl)-5,6,7,8-tetrahydro-2-(((2s)-1-methyl-2-pyrrolidinyl)methoxy)pyrido(3,4-d)pyrimidin-4-yl)-1-(2-fluoro-1-oxo-2-propen-1-yl)-, (2s)-
3. Mrtx-849
4. Mrtx849
1. Mrtx849
2. 2326521-71-3
3. Mrtx-849
4. Adagrasib [usan]
5. Kras G12c Inhibitor Mrtx849
6. 8eoo6hqf8y
7. 2-((s)-4-(7-(8-chloronaphthalen-1-yl)-2-(((s)-1-methylpyrrolidin-2-yl)methoxy)-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-4-yl)-1-(2-fluoroacryloyl)piperazin-2-yl)acetonitrile
8. 2-[(2s)-4-[7-(8-chloronaphthalen-1-yl)-2-[[(2s)-1-methylpyrrolidin-2-yl]methoxy]-6,8-dihydro-5h-pyrido[3,4-d]pyrimidin-4-yl]-1-(2-fluoroprop-2-enoyl)piperazin-2-yl]acetonitrile
9. 2-piperazineacetonitrile, 4-[7-(8-chloro-1-naphthalenyl)-5,6,7,8-tetrahydro-2-[[(2s)-1-methyl-2-pyrrolidinyl]methoxy]pyrido[3,4-d]pyrimidin-4-yl]-1-(2-fluoro-1-oxo-2-propen-1-yl)-, (2s)-
10. 2-piperazineacetonitrile, 4-(7-(8-chloro-1-naphthalenyl)-5,6,7,8-tetrahydro-2-(((2s)-1-methyl-2-pyrrolidinyl)methoxy)pyrido(3,4-d)pyrimidin-4-yl)-1-(2-fluoro-1-oxo-2-propen-1-yl)-, (2s)-
11. Adagrasib [inn]
12. Unii-8eoo6hqf8y
13. Adagrasib [who-dd]
14. Chembl4594350
15. Schembl20974691
16. Gtpl10888
17. Dtxsid801336759
18. Bcp31538
19. Ex-a3258
20. Mrtx-849; Mrtx 849
21. Bdbm50539763
22. Mfcd32263433
23. Nsc831453
24. S8884
25. Who 11519
26. Akos037648997
27. At23561
28. Nsc-831453
29. Compound 20 [pmid: 32250617]
30. Ac-35659
31. Bm177692
32. Bs-16211
33. Hy-130149
34. Cs-0105265
35. A936721
36. ((2s)-4-(7-(8-chloronaphthalen-1-yl)-2-(((2s)-1- Methylpyrrolidin-2-yl)methoxy)-5,6,7,8- Tetrahydropyrido(3,4-d)pyrimidin-4-yl)-1-(2-fluoroprop2-enoyl)piperazin-2-yl)acetonitrile
37. [(2s)-4-[7-(8-chloro-1-naphthyl)-2-{[(2s)-1-methyl-2-pyrrolidinyl]methoxy}-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-4-yl]-1-(2-fluoroacryloyl)-2-piperazinyl]acetonitrile
| Molecular Weight | 604.1 g/mol |
|---|---|
| Molecular Formula | C32H35ClFN7O2 |
| XLogP3 | 5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 7 |
| Exact Mass | 603.2524792 g/mol |
| Monoisotopic Mass | 603.2524792 g/mol |
| Topological Polar Surface Area | 88.8 Ų |
| Heavy Atom Count | 43 |
| Formal Charge | 0 |
| Complexity | 1060 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
MRTX849 is an experimental KRAS inhibitor being investigated for the treatment of KRAS G12C mutant lung and colon adenocarcinomas.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Normally GTP binds to KRAS, activating the protein and promoting effectors to the MAP kinase pathway. GTP is hydrolyzed to GDP, and KRAS is inactivated. KRAS G12C mutations impair hydrolysis of GTP, leaving it in the active form. MRTX849 inhibits KRAS in these types of cancers. This mutation is present in 13% of non small cell lung cancer, 3% of colorectal and appendix cancer, and 1-3% of solid tumors.
 Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.

NDC Package Code : 72015-006
Start Marketing Date : 2022-12-12
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT




 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Adagrasib is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Carcinoma, Non-Small-Cell Lung.
Lead Product(s): Adagrasib,Pembrolizumab,Carboplatin,Pemetrexed,Cisplatin
Therapeutic Area: Oncology Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable March 13, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Adagrasib,Pembrolizumab,Carboplatin,Pemetrexed,Cisplatin
Therapeutic Area : Oncology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Adagrasib is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Carcinoma, Non-Small-Cell Lung.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
March 13, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Adagrasib is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of undefined medical condition.
Lead Product(s): Adagrasib,Inapplicable
Therapeutic Area: Undisclosed Brand Name: Undisclosed
Study Phase: Phase IProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable January 30, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Adagrasib,Inapplicable
Therapeutic Area : Undisclosed
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Bioequivalence Study Between Adagrasib Reference Tablets and High Drug Load Tablets
Details : Adagrasib is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of undefined medical condition.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
January 30, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
KRAZATI (adagrasib), a potent oral small-molecule inhibitor of KRASG12C, is indicated in combination with cetuximab for patients with locally advanced or metastatic colorectal cancer.
Lead Product(s): Adagrasib,Cetuximab
Therapeutic Area: Oncology Brand Name: Krazati
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable June 21, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Adagrasib,Cetuximab
Therapeutic Area : Oncology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Bristol Myers Squibb Announces FDA Approval of KRAZATI with Cetuximab for CRC
Details : KRAZATI (adagrasib), a potent oral small-molecule inhibitor of KRASG12C, is indicated in combination with cetuximab for patients with locally advanced or metastatic colorectal cancer.
Product Name : Krazati
Product Type : Miscellaneous
Upfront Cash : Inapplicable
June 21, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
KRAZATI (adagrasib), a selective oral inhibitor of KRASG12C, is being evaluated with cetuximab for KRASG12C-mutated locally advanced or metastatic colorectal cancer.
Lead Product(s): Adagrasib,Cetuximab
Therapeutic Area: Oncology Brand Name: Krazati
Study Phase: Phase I/ Phase IIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable April 08, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Adagrasib,Cetuximab
Therapeutic Area : Oncology
Highest Development Status : Phase I/ Phase II
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
KRAZATI-Cetuximab Shows Activity for KRAS G12C-Mutated Metastatic Colorectal Cancer
Details : KRAZATI (adagrasib), a selective oral inhibitor of KRASG12C, is being evaluated with cetuximab for KRASG12C-mutated locally advanced or metastatic colorectal cancer.
Product Name : Krazati
Product Type : Miscellaneous
Upfront Cash : Inapplicable
April 08, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
KRAZATI (adagrasib) is a selective oral KRASG12C inhibitor, currently in Phase III trials for treating locally advanced or metastatic non-small cell lung cancer.
Lead Product(s): Adagrasib,Inapplicable
Therapeutic Area: Oncology Brand Name: Krazati
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Bristol Myers Squibb
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable April 01, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Adagrasib,Inapplicable
Therapeutic Area : Oncology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Bristol Myers Squibb
Deal Size : Inapplicable
Deal Type : Inapplicable
Zai Lab and Bristol Myers Squibb Announce KRYSTAL-12 Trial Meets Endpoint
Details : KRAZATI (adagrasib) is a selective oral KRASG12C inhibitor, currently in Phase III trials for treating locally advanced or metastatic non-small cell lung cancer.
Product Name : Krazati
Product Type : Miscellaneous
Upfront Cash : Inapplicable
April 01, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Krazati (adagrasib) is an oral small-molecule KRAS G12C inhibitor under evaluation for treating KRASG12C-mutated advanced/metastatic non-small cell lung cancer.
Lead Product(s): Adagrasib,Inapplicable
Therapeutic Area: Oncology Brand Name: Krazati
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable March 28, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Adagrasib,Inapplicable
Therapeutic Area : Oncology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Bristol Myers Squibb’s KRYSTAL-12 Trial for KRAZATI Meets Primary Endpoint
Details : Krazati (adagrasib) is an oral small-molecule KRAS G12C inhibitor under evaluation for treating KRASG12C-mutated advanced/metastatic non-small cell lung cancer.
Product Name : Krazati
Product Type : Miscellaneous
Upfront Cash : Inapplicable
March 28, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Krazati (adagrasib) is a selective KRAS G12C inhibitor under evaluation for KRAS G12C-mutated metastatic colorectal cancer.
Lead Product(s): Adagrasib,BMS-986466,Cetuximab
Therapeutic Area: Oncology Brand Name: Krazati
Study Phase: Phase I/ Phase IIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable February 20, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Adagrasib,BMS-986466,Cetuximab
Therapeutic Area : Oncology
Highest Development Status : Phase I/ Phase II
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
FDA Accepts KRAZATI® Supplemental NDA for KRAS G12C-Mutated Cancer Treatment
Details : Krazati (adagrasib) is a selective KRAS G12C inhibitor under evaluation for KRAS G12C-mutated metastatic colorectal cancer.
Product Name : Krazati
Product Type : Miscellaneous
Upfront Cash : Inapplicable
February 20, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Adagrasib is a Other Small Molecule drug candidate, which is currently being evaluated in phase II clinical studies for the treatment of Carcinoma, Non-Small-Cell Lung.
Lead Product(s): Adagrasib,Inapplicable
Therapeutic Area: Oncology Brand Name: Undisclosed
Study Phase: Phase IIProduct Type: Miscellaneous
Sponsor: Ryan Gentzler, MD
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable February 08, 2024

Details : Adagrasib is a Other Small Molecule drug candidate, which is currently being evaluated in phase II clinical studies for the treatment of Carcinoma, Non-Small-Cell Lung.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
February 08, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
BMS strengthens and diversifies oncology portfolio by gaining Krazati (adagrasib), a best-in-class KRASG12C inhibitor for advanced non-small cell lung cancer harboring a KRASG12C mutation.
Lead Product(s): Adagrasib,Inapplicable
Therapeutic Area: Oncology Brand Name: Krazati
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Bristol Myers Squibb
Deal Size: $5,800.0 million Upfront Cash: $5,800.0 million
Deal Type: Acquisition January 23, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Adagrasib,Inapplicable
Therapeutic Area : Oncology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Bristol Myers Squibb
Deal Size : $5,800.0 million
Deal Type : Acquisition
Bristol Myers Squibb Completes Acquisition Of Mirati Therapeutics To Strengthen Oncology
Details : BMS strengthens and diversifies oncology portfolio by gaining Krazati (adagrasib), a best-in-class KRASG12C inhibitor for advanced non-small cell lung cancer harboring a KRASG12C mutation.
Product Name : Krazati
Product Type : Miscellaneous
Upfront Cash : $5,800.0 million
January 23, 2024

Details:
Adagrasib is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Neoplasms.
Lead Product(s): Adagrasib,Olaparib
Therapeutic Area: Oncology Brand Name: Undisclosed
Study Phase: Phase IProduct Type: Miscellaneous
Sponsor: Mirati Therapeutics
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable November 14, 2023

Lead Product(s) : Adagrasib,Olaparib
Therapeutic Area : Oncology
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Mirati Therapeutics
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Adagrasib is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Neoplasms.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
November 14, 2023

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]CAS Number : 34381-71-0
End Use API : Adagrasib
About The Company : Founded with a mission to transform strategic capital into specialty chemicals, Ami Group focuses on Agrochemicals, Cosmetics, and Polymers. Ami Organics Ltd. i...

CAS Number : 23356-96-9
End Use API : Adagrasib
About The Company : Anvitha Life Care Private Limited, founded in 2016 by Dr. T. Prakasam and a team of experienced scientists and technocrats with over 80 years of combined expert...

N-Methyl-L-Prolinol / (S)-2-hydroxymethyl-1-methyl...
CAS Number : 34381-71-0
End Use API : Adagrasib
About The Company : Anvitha Life Care Private Limited, founded in 2016 by Dr. T. Prakasam and a team of experienced scientists and technocrats with over 80 years of combined expert...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
43
PharmaCompass offers a list of Adagrasib API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Adagrasib manufacturer or Adagrasib supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Adagrasib manufacturer or Adagrasib supplier.
PharmaCompass also assists you with knowing the Adagrasib API Price utilized in the formulation of products. Adagrasib API Price is not always fixed or binding as the Adagrasib Price is obtained through a variety of data sources. The Adagrasib Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Adagrasib manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Adagrasib, including repackagers and relabelers. The FDA regulates Adagrasib manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Adagrasib API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Adagrasib manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Adagrasib supplier is an individual or a company that provides Adagrasib active pharmaceutical ingredient (API) or Adagrasib finished formulations upon request. The Adagrasib suppliers may include Adagrasib API manufacturers, exporters, distributors and traders.
click here to find a list of Adagrasib suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Adagrasib as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Adagrasib API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Adagrasib as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Adagrasib and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Adagrasib NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Adagrasib suppliers with NDC on PharmaCompass.
Adagrasib Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Adagrasib GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Adagrasib GMP manufacturer or Adagrasib GMP API supplier for your needs.
A Adagrasib CoA (Certificate of Analysis) is a formal document that attests to Adagrasib's compliance with Adagrasib specifications and serves as a tool for batch-level quality control.
Adagrasib CoA mostly includes findings from lab analyses of a specific batch. For each Adagrasib CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Adagrasib may be tested according to a variety of international standards, such as European Pharmacopoeia (Adagrasib EP), Adagrasib JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Adagrasib USP).

