By PharmaCompass
2021-08-25
Impressions: 5914
In 2017, the US Food and Drug Administration (FDA) had announced the Drug Competition Action Plan (DCAP) to encourage robust and timely market competition for generic drugs. The objective of the DCAP was to bring about greater efficiency and transparency to the generic drug review process, without sacrificing the scientific rigor underlying the agency’s generic drug program.
Since then, the FDA has been publishing a list of off-patent, off-exclusivity drugs without an approved generic. The list gets updated every six months. With this list, the FDA hopes to bolster competitiveness in the generics market. Such updates are a part of the FDA’s initiative to improve transparency and encourage the development and submission of abbreviated new drug applications (ANDAs) in markets with little competition.
In September last year, PharmaCompass had reviewed the June 2020 list, and this week we bring you the key highlights of the June 2021 list.
View FDA's List of Off-Patent, Off-Exclusivity Drugs with No Approved Generics
Thirty-five new entries in latest list
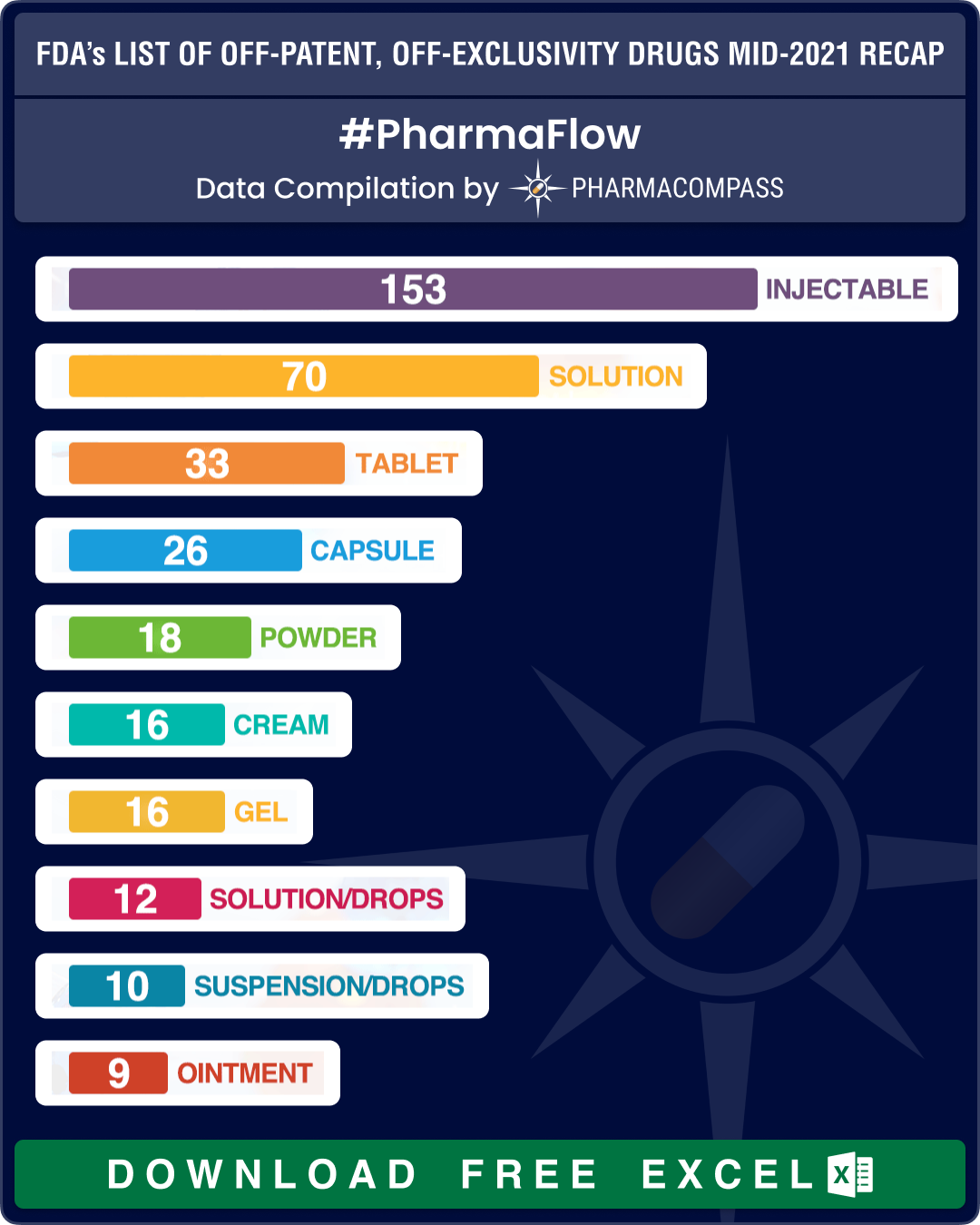
The FDA updated its list of ‘off-patent, off-exclusivity drugs without an approved generic’ in June this year. The latest compilation contains 451 entries with 311 being classified as Part I (drug products for which FDA could immediately accept an ANDA without prior discussion), 133 as Part II (drug products for which ANDA development or approval may raise potential legal, regulatory, or scientific issues that should be addressed with the FDA prior to considering submission of an ANDA) and seven products being added to the Appendix, which indicates one or more ANDAs referencing such NDA (new drug application) drug products have been approved since the publication of the previous list.
Since our last publication a year ago, there are 35 new entries in the list which include the anti-hypertensive levamlodipine maleate tablets, heparin sodium injections and many other new drugs and drug products.
Almost one-third of the products – 153 out of 451 – continue to be drug products delivered as injectables, while a little over 70 entries on the list are delivered as oral solid dosage forms (such as tablets, capsules, modified release forms, etc.).
View FDA's List of Off-Patent, Off-Exclusivity Drugs with No Approved Generics
FDA approves Viatris-Biocon’s biosimilar insulin
As part of the initiative to approve generics and increase the availability of medicines, earlier this month, the FDA approved the first interchangeable biosimilar insulin product – Semglee – indicated to improve glycemic control in adults and pediatric patients with Type 1 and Type 2 diabetes mellitus.
Semglee (insulin glargine-yfgn) is both biosimilar to, and interchangeable with (can be substituted for), its reference product Lantus (insulin glargine), a long-acting insulin analog.
Semglee has been developed by Viatris Inc and Biocon Biologics (a subsidiary of Indian drugmaker Biocon Ltd).
“This is a momentous day for people who rely daily on insulin for treatment of diabetes, as biosimilar and interchangeable biosimilar products have the potential to greatly reduce healthcare costs,” said acting FDA commissioner, Janet Woodcock. “Today’s approval of the first interchangeable biosimilar product furthers FDA’s longstanding commitment to support a competitive marketplace for biological products and ultimately empowers patients by helping to increase access to safe, effective and high-quality medications at potentially lower cost.”
View FDA's List of Off-Patent, Off-Exclusivity Drugs with No Approved Generics
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : FDA's-List-of-Off-Patent-Off-Exclusivity-Drugs-with-No-Approved-Generics by PharmaCompass is licensed under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”







