Now that it has been
established that the novel coronavirus is going to globally impact the drug
supply chain, it becomes imperative to analyze the extent of the impact.
Since the outbreak of
the novel coronavirus — COVID-19 — in December, PharmaCompass has been constantly reaching out to
manufacturers around the world to assess the current state of the drug supply
chain. This week, we share our preliminary analysis based on the feedback we
have received from drug manufacturers around the world.
Drug shortages are
for real
Last week, the US
Food and Drug Administration (FDA) announced the first human drug shortage
as a result of the coronavirus outbreak. In addition, the FDA announced it was
tracking 20 drugs that could face shortages. Some generic drugmakers are predicting shortages
as early as in June or July, due to the novel coronavirus.
The FDA did not disclose the name of the drug in shortage or the 20 drugs it is tracking, as this is considered ‘confidential commercial information’.
In India, a committee constituted by the country’s Department of Pharmaceuticals started monitoring the availability of 58 active pharmaceutical ingredients (APIs) to take preventive measures
against illegal hoarding and black-marketing in the country.
According to a report published in The Economic Times, after
reviewing the list of drugs, 34 were found to have no alternatives which
include critical and essential drugs like potassium clavulanate, ceftriaxone sodium sterile, piperacillin tazobactam, meropenem, vancomycin, gentamycin and ciprofloxacin.
This was immediately
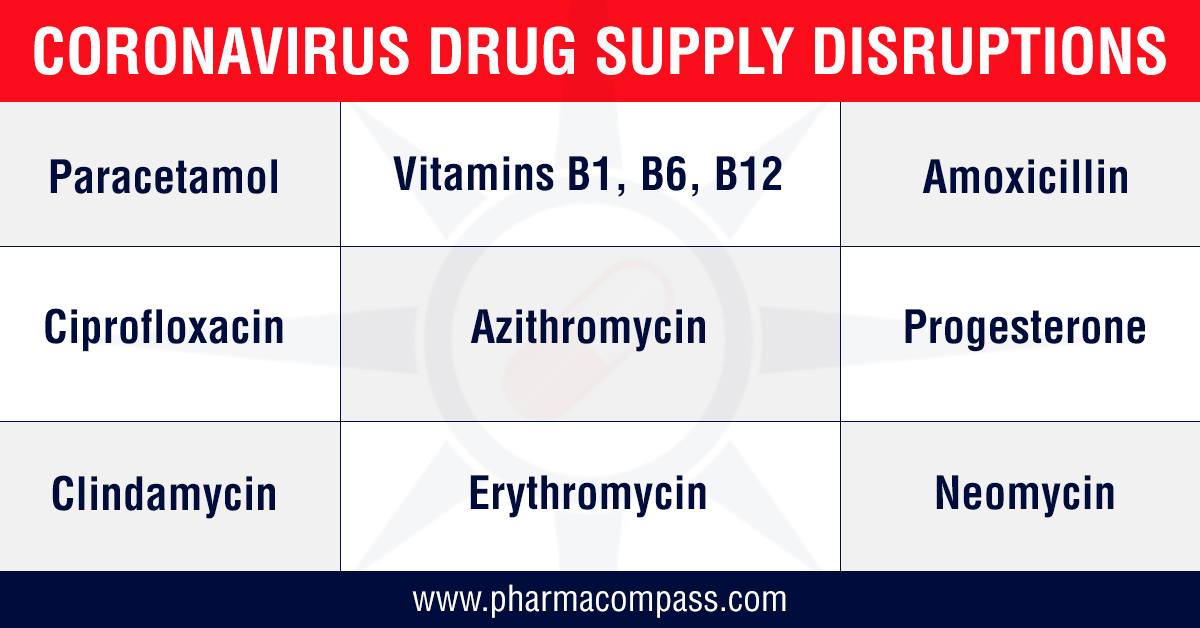
followed by the Indian government restricting the exports of 13
APIs along with some of their finished formulations. The list includes paracetamol, tinidazole, metronidazole, acyclovir, vitamin B1, vitamin B6, vitamin B12, progesterone, chloramphenicol and neomycin. For most
of the products on this list, India is a net importer, as there is little
domestic manufacturing of these APIs.
COVID-19 is also
likely to impact bottomlines. Leading generic drugmaker Mylan said it expects the coronavirus outbreak to impact its financial results
while some of the largest drugmakers — including AstraZeneca, Merck and Pfizer — have said that the coronavirus outbreak could affect their supplies or sales.
Paracetamol
affected; prices double in less regulated markets
The decline in industrial activity in China is certainly taking its toll, as drugs which are on the World Health Organization’s Model list of Essential Medicines are beginning to face significant price increases in the wake of disruption of key starting raw materials for bulk drugs.
The export
restriction out of India on commonly used analgesic, Paracetamol — sold under the brand names such as Tylenol (in the US), Panadol (in the UK), Dafalgan (France) and Crocin
(India) — is not surprising as the API has witnessed almost doubling of prices in less regulated markets because exports of its key building block para-amino phenol (PAP) have dramatically reduced from China.
While there are only
a few manufacturers who produce paracetamol without being dependent on Chinese
PAP, a few major manufacturers in India depend almost completely on Chinese PAP
for their paracetamol production and usually only keep three to four months of
inventory.
By the end of
February, their inventory stockpiles had halved and in the event of a continued
supply disruption, their entire inventory pipeline is likely to dry out. In
addition, Chinese paracetamol manufacturers, who export a significant amount of
their bulk ingredient production globally, including to India, are also
currently unable to export. This is beginning to create the potential of panic
among sourcing executives across the world.
Several
antibiotics also in danger of acute shortages
While paracetamol was listed on the API watch list circulated by India’s Department of Pharmaceuticals, our survey has revealed that other products on the list like ciprofloxacin, amoxicillin and azithromycin are also facing severe raw material
shortages. As a result, the prices of these bulk drugs have also increased
sharply.
In a statement to The Economic Times, leading Indian generic manufacturer Mankind Pharma’s chairman and managing director said
amoxicillin is the most commonly used API to manufacture antibiotics and the
company has invested Rs 1 billion (US$ 14 million) in placing irregular orders
with vendors to try and address the potential shortage that is expected. He
went on to say that if the situation continues until April, there will be an
acute shortage.
In a statement to the US House of Representatives last October, Janet Woodcock, the FDA’s Director of Center of Drug Evaluation and Research, said the FDA has determined that there are three WHO Essential Medicines whose API manufacturers are based only in China. The three medicines are: capreomycin, streptomycin (both indicated to treat Mycobacterium
tuberculosis) and sulfadiazine (used to treat chancroid and trachoma).
Streptomycin is also on the watch list published by India’s Department of Pharmaceuticals along with commonly used anti-hypertensives like losartan, valsartan, telmisartan and olmesartan and diabetes treatment metformin.
Intermediates
becoming a problem for generic drugmakers
PharmaCompass’ discussions have also revealed that in many cases while API manufacturing factories in China have returned to work, there are disruptions in the availability of raw materials and/or logistics at sea ports and airports which have led to unavailability of supplies.
While the FDA has a
list of the number of API facilities in China which are in a position to supply
to the United States, Woodcock said in her statement that the FDA “cannot determine with any precision the volume of API that China is actually producing, or the volume of APIs manufactured in China that is entering the US market.”
This visibility
reduces drastically when one has to assess the dependence of each API
manufacturer around the world on China for intermediates. Our discussions have
revealed that it is these intermediates which are becoming a problem for most
API manufacturers, even those based in India.
It was worth
highlighting that a manufacturing process change at an intermediate stage of
commonly used blood pressure medicine valsartan resulted in the recall of
millions of pills as it was found to contain a cancer causing impurity above
acceptable levels. Similarly, in 2008, the adulteration of heparin in China,
which killed 81 people and left 785 severely injured, was an outcome of the
subcontracting of precursor chemicals of Heparin.
Our view
The over-dependence
on China for key starting materials has been the subject of discussion ever
since we launched PharmaCompass. Rosemary Gibson explored this subject
in detail in her book China Rx: Exposing the Risks of America’s Dependence on
China for Medicine.
The restrictions imposed on industrial activity and transportation in China in the first two months of this year has resulted in NASA’s satellite images showing a decline in pollution levels over China.
While China works
towards getting its industrial and transportation engine up and running to 2019
levels, the outbreak has spread to other countries which will further increase
the demand for drugs to fight the virus.
This is a time when
the pharmaceutical industry needs to act responsibly and make decisions which
are in the best interests of patients globally.
Sharing information is one such step — it will allow for drug stockpiles and inventories that exist to be re-distributed to areas which need them most. For, in the event of an urgent need, drugs will become available to those who are most in need.
Impressions: 8184
In April 2016, the US Food and Drug Administration (FDA) came
down heavily on Semler Research Center over issues of data manipulation. The
FDA had told drug firms that their applications seeking
approvals on the basis of studies done by the Bangalore-based firm will not
be accepted. It had also asked firms to furnish additional clinical research from
other approved entities to get the FDA nod.The action had been taken as a result of an inspection of
Semler's bioanalytical facility in Bangalore conducted between September 29,
2015, and October 9, 2015. Generics relying on
data from Semler are not considered equivalent to the brand The FDA also changed the therapeutic equivalence (TE) rating in the Orange Book (also called the Approved Drug Product with Therapeutic Equivalence Evaluations) for any approved ANDA that relied on data from Semler to “BX.” A BX rating indicates that data reviewed by the agency are
insufficient to determine therapeutic equivalence, i.e., substitutability, of
the generic product to the drug it references.As drug regulators across the world invalidate clinical
studies conducted at Semler Research that demonstrate equivalence of the
generic drugs to branded products, PharmaCompass
brings to you the impact of this scandal on various products and drug companies.
Dr. Reddy’s generic Nexium gains as competitor gets impactedThe violations uncovered at Semler Research have impacted
the global generic pharmaceutical business. While most companies have been
adversely impacted by the Semler data integrity scandal, there are some that
have gained as well. For instance, Dr. Reddy’s North American business has got an unanticipated
sales boost due to the
issues at Semler. This was because the FDA mandated that competitor Hetero’s
generic Nexium
(an acid reflux medication) repeat its bioequivalence trials to be considered
as an equivalent generic of the brand drug. This reclassification of Hetero’s drug has increased market share gains for Dr. Reddy’s Esomeprazole
Magnesium – a generic equivalent of Nexium launched in the US in September last year. 96 European marketing
authorizations to get impactedPharmaCompass’ assessment has uncovered 96
marketing authorizations in Europe for which “clinical and bioanalytical parts of the bioequivalence studies were performed at the Semler Research Center (SRC)”. Of these, 20 marketing authorizations are in France alone, followed by 10 each in Germany, the Netherlands and the United Kingdom. Click here to receive your copy of the European Marketing
Authorizations Landscape due to the data-integrity violations at Semler A marketing authorization application is an application
submitted by a drug manufacturer seeking permission to bring a newly developed
medicine or a medicinal product to the market.In all probability, the maximum fallout of the Semler
episode will be on Sandoz, as 29
marketing authorizations of Sandoz are likely to be recommended for repeat
studies by authorities. Other generic majors who will possibly repeat studies are Mylan (15
marketing authorizations), Teva
(nine marketing authorizations), Ratiopharm
(six marketing authorizations) and Venipharm (five marketing
authorizations). Changes in therapeutic status of Hetero, Lupin’s drugsWhile Hetero has to repeat its studies for generic Nexium in
the United States, six Hetero filings in Europe have been listed by authorities
for which the clinical studies were conducted at Semler.In the United States, the FDA has also changed the therapeutic
status of Hetero’s Losartan
Potassium along with Lupin’s filing
for the same product to one (i.e. BX) where the product is no longer considered
equivalent to the brand. Lupin’s Azithromycin,
Upsher-Smith’s
Propranolol
Hydrochloride and Unique Pharma’s Tinidazole
are other products which have seen their therapeutic code category get changed (to
one of not being bioequivalent) by the FDA in the past month.Click here to receive your copy of the European Marketing
Authorizations Landscape due to the data-integrity violations at Semler WHO questions
findings of Semler studiesWhile the FDA and European regulators are busy dealing with
the aftermath of the problems at Semler, the World Health Organization (WHO) has
also been very active. In the Notice
of Concern (NOC) issued by the WHO to Semler, as an outcome of WHO’s own inspections and discussions, Semler acknowledged that “four FDA studies and one WHO study have questionable data”. The WHO recommended “an immediate stop for all submissions of dossiers relying in whole or in part on involvement from Semler”. The WHO has questioned the findings of 11 studies performed at Semler for products which meet WHO’s pre-qualification criteria. The studies were performed on behalf of Mylan (three studies),
Lupin (five studies), Micro Labs
(one study) and Strides
Ltd (two studies). Additionally, the WHO has also revealed 12 studies for
which the products are currently under assessment but not yet pre-qualified. Our viewSemler’s data integrity concerns have made drug regulators question the equivalency of over 110 generic drug applications. Concerns have been highlighted by the FDA, European Medicines Agency (EMA) and the WHO. And the steps taken by the regulators indicate the magnitude of the fallout of these inspections. For the generic pharmaceutical industry, life has become a
lot more challenging. In addition to concerns about in-house manufacturing
compliance problems, they also need to worry about data integrity issues at
clinical research firms. Clinical
trial falsification issues at the laboratories of Quest Life Sciences, GVK
Biosciences, Alkem
Laboratories and Semler indicate that a sustained supply of generics can no
longer be taken for granted.Click here to receive your copy of the European Marketing
Authorizations Landscape due to the data-integrity violations at Semler
Impressions: 5652