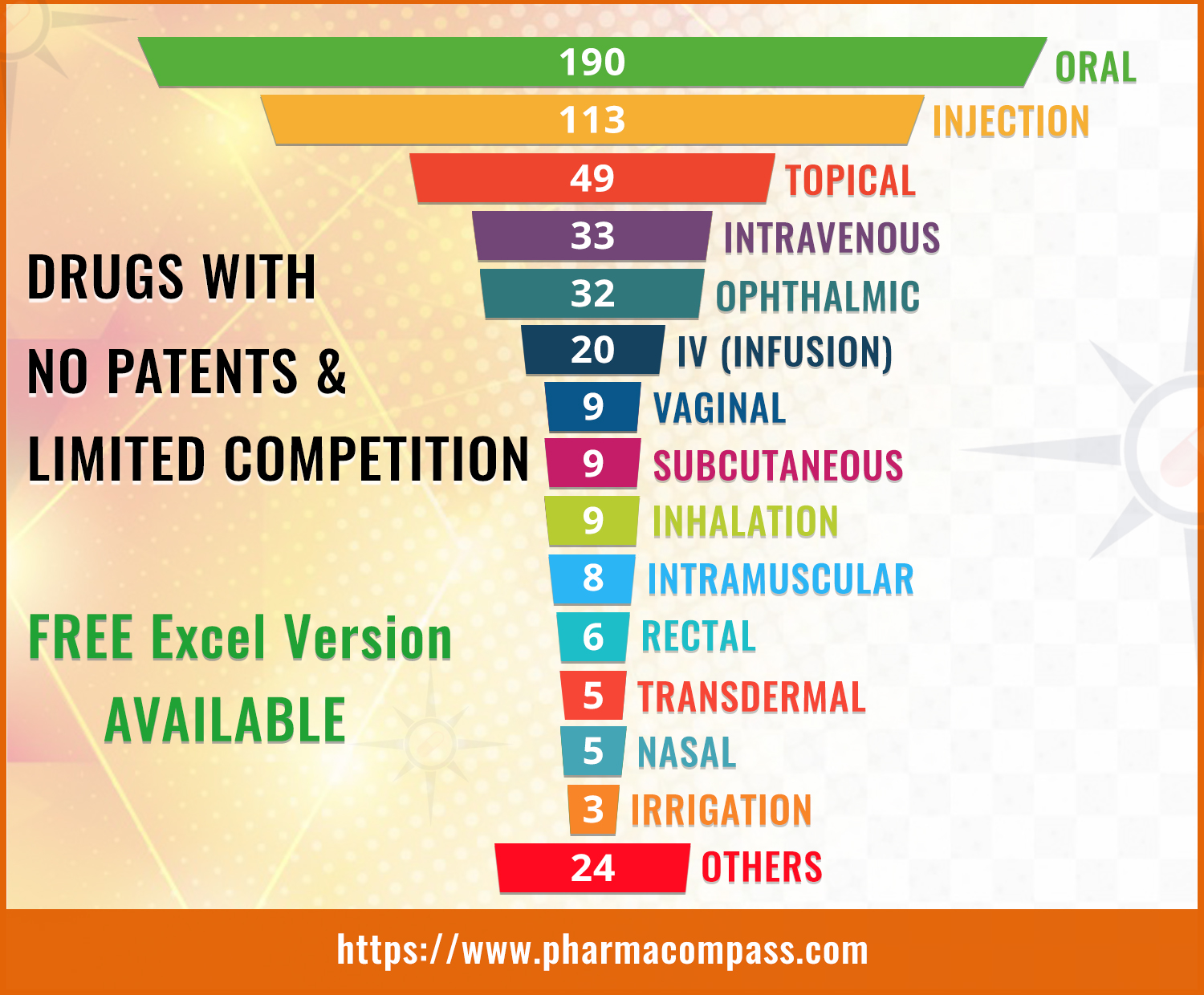
In April last year, PharmaCompass had shared a list of over 300 different dosage forms of drugs which had no patents and no competition.
These drugs were ripe for ‘price-gouging’ — a hot industry topic that became synonymous with activities of many major pharmaceutical companies, after Martin Shkreli (former CEO of Turing Pharmaceuticals) increased the price of Daraprim (Pyrimethamine) from US$ 13.50 per tablet to US$ 750 overnight in the US. Similarly, Valeant Pharmaceuticals adopted a strategy of buying up companies and dramatically increasing the price of the acquired drugs.
Although it has been a while since Hillary Clinton tweeted angrily: “Price gouging like this in the specialty drug market is outrageous”; last week newly appointed US Food Drug Administration (FDA) Commissioner Scott Gottlieb, indicated that the topic is still very much on top of his agenda.
FDA to play a more active
role in drug pricing
On May 25, Gottlieb told a congressional panel that the FDA is developing a “drug competition action plan” aimed at expediting approval of generic versions of brand-name medications that lack competition.
“While FDA does not play a direct role in drug pricing, we can take steps to facilitate entry of lower-cost alternatives to the market and increase competition,” Gottlieb said in his testimony before a House Appropriations subcommittee. “This is especially true when it comes to safe and effective generic medicines.”
In his testimony, Gottlieb highlighted that he would like the FDA to take a more active role in pricing and would publish a list of drugs that are off-patent and lack generic competition.
Our compilation — Drugs with No Patents and No Competition
500 dosage forms this year, where oral drugs (tablets, capsules etc.) had the least competition followed by drugs administered as injections.
The list has over 400 different active pharmaceutical ingredients (APIs) that are used in manufacturing these dosage forms.
While there are almost 70 drugs which were approved recently (since 2015), the majority of products on the list are those that have been on the market for years.
Some of the products that made it to our list last year continue to enjoy monopolies, such as Pfizer's Premarin, isolated from pregnant mares’ urine, which brings in over US$ 1 billion in sales to Emcure’s BICNU (carmustine or BICNU is an anti-cancer, chemotherapy drug).
While Emcure is still banned from exporting products to the United States, due to compliance problems found in its manufacturing operations, BICNU remains exempted since there is no other alternative available in the market.
While an estimated 13 million people in the United States have latent tuberculosis infection (LTBI), Sanofi’s Priftin (rifapentine) still has no generic competition.
Our view
Our list provides tremendous opportunities for generic companies in the short-term. In many cases, to capitalize on the opportunities, generic drug companies will need to partner with API manufacturers as the lack of sustained, quality API supply is a major reason that there are no competitors on the market.
However, it is important for generic companies to remember that the FDA’s continued focus on accelerating review of these drugs will require companies to rely on strategies less opportunistic than price-gouging, to drive their future business growth.
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : Drugs With No Patents & Limited Competition by PharmaCompass is licensed under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”





