Acquisitions and spin-offs dominated headlines in 2019 and the tone was set very early with Bristol-Myers Squibb acquiring
New Jersey-based cancer drug company Celgene in a US$ 74 billion deal announced on
January 3, 2019. After factoring
in debt, the deal value ballooned to about US$ 95 billion, which according
to data compiled by Refinitiv, made it the largest healthcare deal on
record.
In the summer, AbbVie Inc,
which sells the world’s best-selling drug Humira, announced its acquisition of Allergan Plc, known for Botox and other cosmetic
treatments, for US$ 63 billion. While the companies are still awaiting
regulatory approval for their deal, with US$ 49 billion in combined 2019
revenues, the merged entity would rank amongst the biggest in the industry.
View Our Interactive Dashboard on Top drugs by sales in 2019 (Free Excel Available)
The big five by pharmaceutical sales — Pfizer,
Roche, J&J, Novartis and Merck
Pfizer
continued
to lead companies by pharmaceutical sales by reporting annual 2019 revenues of
US$ 51.8 billion, a decrease of US$ 1.9 billion, or 4 percent, compared to
2018. The decline was primarily attributed to the loss of exclusivity of Lyrica in 2019,
which witnessed its sales drop from US$ 5 billion in 2018 to US$ 3.3 billion in
2019.
In 2018, Pfizer’s then incoming CEO Albert Bourla had mentioned that the company did not see the need for any large-scale M&A activity as Pfizer had “the best pipeline” in its history, which needed the company to focus on deploying its capital to keep its pipeline flowing and execute on its drug launches.
Bourla stayed true to his word and barring the acquisition of Array Biopharma for US$ 11.4 billion and a spin-off to merge Upjohn, Pfizer’s off-patent branded and generic established medicines business with
Mylan, there weren’t any other big ticket deals which were announced.
The
Upjohn-Mylan merged entity will be called Viatris and is expected to have 2020
revenues between US$ 19 and US$ 20 billion
and could outpace Teva to
become the largest generic company in the world, in term of revenues.
Novartis, which had
followed Pfizer with the second largest revenues in the pharmaceutical industry
in 2018, reported its first full year earnings after spinning off its Alcon eye
care devices business division that
had US$ 7.15 billion in 2018 sales.
In 2019,
Novartis slipped two spots in the ranking after reporting total sales of US$
47.4 billion and its CEO Vas Narasimhan continued his deal-making spree by buying New
Jersey-headquartered The Medicines Company (MedCo) for US$ 9.7
billion to acquire a late-stage cholesterol-lowering
therapy named inclisiran.
As Takeda Pharmaceutical Co was
busy in 2019 on working to reduce its debt burden incurred due to its US$ 62
billion purchase of Shire Plc, which was announced in 2018, Novartis also purchased
the eye-disease medicine, Xiidra, from the Japanese drugmaker for US$ 5.3 billion.
Novartis’ management also spent a considerable part of 2019 dealing with data-integrity concerns which emerged from its 2018 buyout of AveXis, the
gene-therapy maker Novartis had acquired for US$ 8.7 billion.
The deal gave Novartis rights to Zolgensma,
a novel treatment intended for children less than two years of age with the
most severe form of spinal muscular atrophy (SMA). Priced at US$ 2.1 million,
Zolgensma is currently the world’s most expensive drug.
However,
in a shocking announcement, a month after approving the drug, the US Food and
Drug Administration (FDA) issued a press release on
data accuracy issues as the agency was informed by AveXis that
its personnel had manipulated data which
the FDA used to evaluate product comparability and nonclinical (animal)
pharmacology as part of the biologics license application (BLA), which was
submitted and reviewed by the FDA.
With US$
50.0 billion (CHF 48.5 billion) in annual pharmaceutical sales, Swiss drugmaker
Roche came in at number two position in 2019
as its sales grew 11 percent driven by
its multiple sclerosis medicine Ocrevus, haemophilia drug Hemlibra and cancer medicines Tecentriq and Perjeta.
Roche’s newly introduced medicines generated US$ 5.53 billion (CHF 5.4 billion) in growth, helping offset the impact of the competition from biosimilars for its three best-selling drugs MabThera/Rituxan, Herceptin and Avastin.
In late 2019, after months of increased
antitrust scrutiny, Roche completed
its US$ 5.1 billion acquisition of Spark Therapeutics to strengthen its presence in
gene therapy.
Last year, J&J reported almost flat worldwide sales of US$ 82.1 billion. J&J’s pharmaceutical division generated US$ 42.20 billion and its medical devices and consumer health divisions brought in US$ 25.96 billion and US$ 13.89 billion respectively.
Since J&J’s consumer health division sells analgesics, digestive health along with beauty and oral care products, the US$ 5.43 billion in consumer health sales from over-the-counter drugs and women’s health products was only used in our assessment of J&J’s total pharmaceutical revenues. With combined pharmaceutical sales of US$ 47.63 billion, J&J made it to number three on our list.
While the sales of products like Stelara, Darzalex, Imbruvica, Invega Sustenna drove J&J’s pharmaceutical business to grow by 4 percent over 2018, the firm had to contend with generic competition against key revenue contributors Remicade and Zytiga.
US-headquartered Merck, which is known as
MSD (short for Merck Sharp & Dohme) outside the United States and
Canada, is set to significantly move up the rankings next year fueled by its
cancer drug Keytruda, which witnessed a 55
percent increase in sales to US$ 11.1 billion.
Merck reported total revenues of US$ 41.75 billion and also
announced it will spin off its women’s health drugs,
biosimilar drugs and older products to create a new pharmaceutical
company with US$ 6.5 billion in annual revenues.
The firm had anticipated 2020 sales between US$ 48.8 billion and US$ 50.3 billion however this week it announced that the coronavirus pandemic will reduce 2020 sales by more than $2 billion.
View Our Interactive Dashboard on Top drugs by sales in 2019 (Free Excel Available)
Humira holds on to remain world’s best-selling drug
AbbVie’s acquisition of Allergan comes as the firm faces the expiration of patent protection for Humira, which brought in a staggering US$ 19.2 billion in sales last year for
the company. AbbVie has failed to successfully acquire or develop a major new
product to replace the sales generated by its flagship drug.
In 2019, Humira’s US revenues increased 8.6 percent to US$ 14.86 billion while internationally, due
to biosimilar competition, the sales dropped 31.1 percent to US$ 4.30 billion.
Bristol Myers Squibb’s Eliquis, which is also marketed by Pfizer, maintained its number two position
and posted total sales of US$ 12.1 billion, a 23 percent increase over 2018.
While Bristol Myers Squibb’s immunotherapy treatment Opdivo, sold in partnership with Ono in Japan, saw sales increase from US$ 7.57 billion to US$ 8.0 billion, the growth paled in comparison to the US$ 3.9
billion revenue increase of Opdivo’s key immunotherapy competitor Merck’s Keytruda.
Keytruda took the number three spot in drug sales that
previously belonged to Celgene’s Revlimid, which witnessed a sales decline from US$ 9.69 billion to US$ 9.4 billion.
Cancer treatment Imbruvica, which is marketed
by J&J and AbbVie, witnessed a 30 percent increase in sales. With US$ 8.1
billion in 2019 revenues, it took the number five position.
View Our Interactive Dashboard on Top drugs by sales in 2019 (Free Excel Available)
Vaccines – Covid-19 turns competitors into partners
This year has been dominated by the single biggest health emergency in years — the novel coronavirus (Covid-19) pandemic. As drugs continue to fail to meet expectations, vaccine development has received a lot of attention.
GSK reported the highest vaccine sales of all drugmakers with
total sales of US$ 8.4 billion (GBP 7.16 billion), a significant portion of its
total sales of US$ 41.8 billion (GBP 33.754 billion).
US-based Merck’s vaccine division also reported a significant increase in sales to US$ 8.0 billion and in 2019 received FDA and EU approval to market its Ebola vaccine Ervebo.
This is the first FDA-authorized vaccine against the deadly virus which causes
hemorrhagic fever and spreads from person to person through direct contact with
body fluids.
Pfizer and Sanofi also reported an increase in their vaccine sales to US$ 6.4
billion and US$ 6.2 billion respectively and the Covid-19 pandemic has recently
pushed drugmakers to move faster than ever before and has also converted
competitors into partners.
In a rare move, drug behemoths — Sanofi and GlaxoSmithKline (GSK) —joined hands to develop a vaccine for the novel coronavirus.
The two companies plan to start human trials
in the second half of this year, and if things go right, they will file
for potential approvals by the second half of 2021.
View Our Interactive Dashboard on Top drugs by sales in 2019 (Free Excel Available)
Our view
Covid-19 has brought the world economy to a grinding halt and shifted the global attention to the pharmaceutical industry’s capability to deliver solutions to address this pandemic.
Our compilation shows that vaccines and drugs
for infectious diseases currently form a tiny fraction of the total sales of
pharmaceutical companies and few drugs against infectious diseases rank high on
the sales list.
This could well explain the limited range of
options currently available to fight Covid-19. With the pandemic currently infecting
over 3 million people spread across more than 200 countries, we can safely
conclude that the scenario in 2020 will change substantially. And so should our
compilation of top drugs for the year.
View Our Interactive Dashboard on Top drugs by sales in 2019 (Free Excel Available)
Impressions: 54752
If
the first two months of 2018 saw pharma and biotech firms receive more money
than what
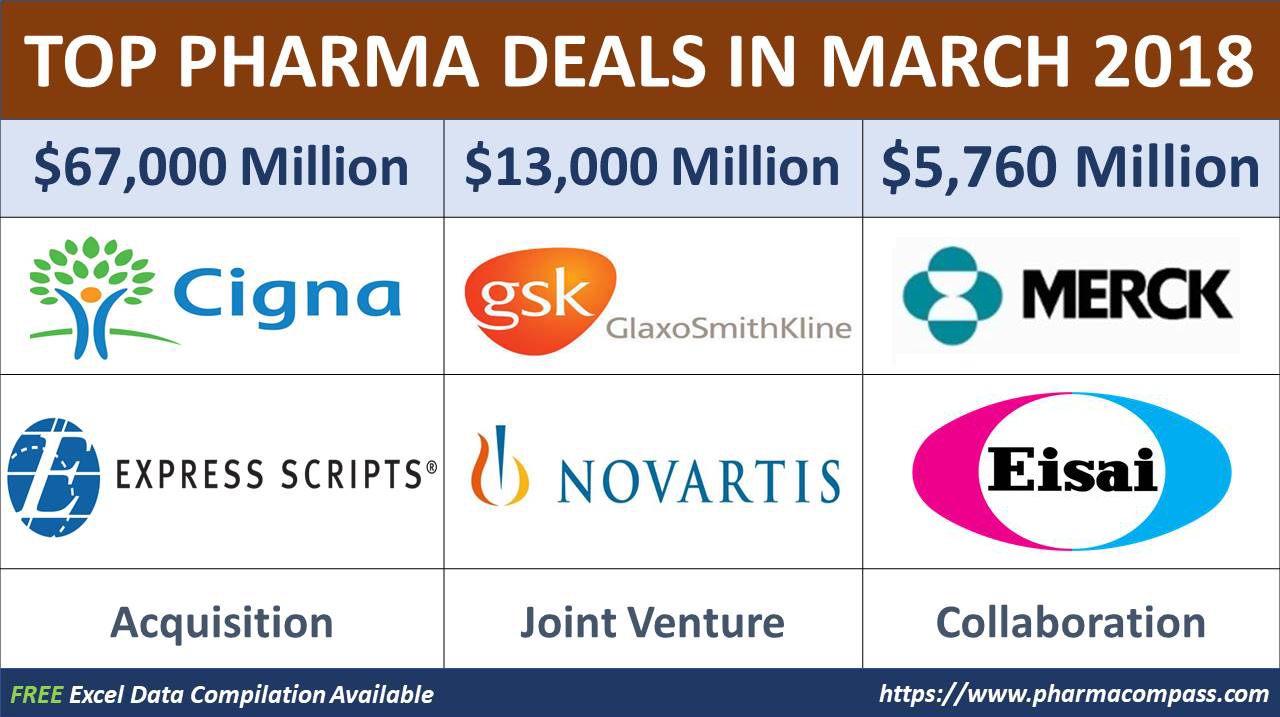
all biotech companies raised throughout 2013, March witnessed the biggest takeover transaction of the year so far — health insurer Cigna Corporation acquired pharmacy benefit manager (PBM) Express Scripts Holding for around US$ 67 billion.
Healthcare shakeout
in the United States continues
As
part
of a shakeout in
healthcare that has indeed gathered momentum in the United
States, primarily in response to the growing frustration over drug pricing, health insurer Cigna Corporation has agreed to buy pharmacy benefit manager (PBM) Express Scripts
Holding for around US$ 67 billion.
Click here to view the major deals in March 2018
(FREE Excel version available)
As
of 2017, Express Scripts is the largest of the remaining independent PBMs. The
deal would give the two companies substantial bargaining power over drug prices
in the US.
Healthcare
spending has been rising rapidly, accounting for an estimated 18 percent of the
US economy in 2017. PBMs, such as Express Scripts, negotiate drug benefits for
insurance plans and employers.
The medical supply chain has become cumbersome. Insurers, PBMs, drug distributors, pharmacies, and large medical groups — all get a cut of the profits from caring for patients. Bringing these businesses under one roof could streamline costs and improve care.
The
deal could help Cigna compete with players like CVS Health Corp and
UnitedHealth Group Inc. The former recently acquired Aetna Inc. for around US$ 69 billion, linking its pharmacies and drug-benefit plans with the insurer’s coverage.
There are lots happening in the PBM space. In March, Andrew Witty, former CEO of GlaxoSmithKline who
retired from the British drug major a year back, announced he was taking a leadership role
in managing drug benefits in one of the largest, fastest growing outfits in the
US.
Click here to view the major deals in March 2018
(FREE Excel version available)
Witty has been named the new CEO of UnitedHealth’s Optum division, a PBM group and healthcare analytics company. Optum has 140,000 staffers around the world. It earns roughly half of UnitedHealth’s revenue (which was US$ 201 billion in 2017) from its three key subsidiaries — OptumHealth and OptumInsight as well as OptumRx.
Merck continues its
cancer drug deal making
Japan’s Eisai Co and
US-based Merck & Co announced
a potential multibillion-dollar deal to develop and sell Eisai’s cancer drug Lenvima, which is already
approved in dozens of countries as treatment for thyroid cancer and advanced
kidney cancer (when used along with another medicine).
As
per the deal, the Japanese drugmaker could potentially receive up to US$ 5.76 billion if it proves a success. This includes an
upfront payment of US$ 750 million. The remaining US$ 5.01 billion will be paid
by Merck for development achievements and sales milestones, a joint statement
said.
Merck
will be entitled to half of all global Lenvima sales revenue, even for its
already approved uses for thyroid cancer and advanced kidney cancer.
The
deal is similar to a
multibillion-dollar oncology collaboration Merck struck with AstraZeneca Plc for
its cancer drug Lynparza last year.
Click here to view the major deals in March 2018
(FREE Excel version available)
Ionis buys back into company it spun-off
less than a year ago
Akcea Therapeutics, a biotech company focused on rare
diseases caused by lipid disorders, was founded in 2015 as a
subsidiary of Ionis Pharmaceuticals.
Its drug pipeline of four drug candidates were developed using Ionis’ technology and its most advanced drug was volanesorsen, a drug
developed to treat familial partial lipodystrophy, or FPL.
A year ago, Akcea was spun out of its
parent as it filed an initial public stock offering (IPO) to drive forward
the FDA approval for its lead drug which is expected by mid-2018.
Last month, Ionis announced that it
has signed an exclusive, worldwide licensing deal with Akcea related to
two of its other drugs - inotersen and AKCEA-TTR-LRx, formerly
IONIS-TTR-LRx.
The total milestone payments associated with
this deal are $1.3 billion while the
transaction could make Ionis eligible for up to $1.7
billion, plus profit-sharing payments.
Click here to view the major deals in March 2018
(FREE Excel version available)
Interim Lundbeck CEO’s billion-dollar buy
Denmark-based H. Lundbeck announced
it was acquiring Prexton Therapeutics for US$ 123 million. Lundbeck is also committing an additional US$ 1 billion in milestones — with more than half of that tied to sales goals.
Prexton Therapeutics was formed as a spin-off from Merck in 2012.
The acquisition has a mid-stage Parkinson’s drug— foliglurax — at its core. The drug is in Phase II testing for the symptomatic treatment of Parkinson’s disease and dyskinesia, including Levodopa Induced Dyskinesia (LID). First
data from the ongoing clinical Phase II program is expected to be available in
the first half of 2019, the company said.
Click here to view the major deals in March 2018
(FREE Excel version available)
Anders Götzsche, the interim CEO of Lundbeck, said the acquisition will provide Lundbeck full control of the future of foliglurax. “Foliglurax addresses high unmet needs with its potential indication in Parkinson’s fitting perfectly within Lundbeck’s core areas and this treatment option also appears to be highly interesting for patients, physicians and payers,” Götzsche said.
Click here to view the major deals in March 2018
(FREE Excel version available)
The Phase II trial, which began in 2017, will add foliglurax to standard care of treatment for Parkinson’s disease, which includes drugs like levodopa. The primary goal of the Phase II trial is to assess efficacy, safety and tolerability of foliglurax in reducing motor complications of levodopa therapy in patients experiencing end-of-dose wearing-off and LID.
GlaxoSmithKline
doubles down on OTC
Over the past few months, there has been a lot of speculation around who will acquire Pfizer’s consumer
health division auction has been the subject of a lot of speculation over the
past few months. Last month Reckitt Benckiser pulled out of the
race and British drug major GlaxoSmithKline was considered the leader in the race for the
division. However, GSK too withdrew from the deal talks.
While there was news that Glaxo had made a final bid valuing Pfizer’s over-the-counter (OTC) treatments at about US$
15 billion to US$ 20 billion, GSK announced
it had reached an agreement with Novartis to acquire full ownership of
its consumer healthcare business.
GSK will buy out Novartis’ 36.5 percent stake in a joint venture worth US$ 13 billion (£9.2 billion).
Click here to view the major deals in March 2018
(FREE Excel version available)
With this acquisition, GSK is in full control of a joint venture that owns successful OTC
products like Sensodyne toothpaste, Panadol headache tablets and Nicotinell patches.
In 2017, the business reported sales of US$ 11 billion (£7.8 billion).
Japan’s Fujifilm bets on the market for cell culture media
Irvine Scientific Sales Company
(ISUS) and IS Japan (ISJ), two companies specializing in cell culture media were
acquired last month for $800 million by Japan’s Fujifilm.
In recent years, culture media has received increased
interests as it contains the nutrients required for the growth and
proliferation of cells essential for cell culturing in the R&D and
manufacturing of biopharmaceuticals and regenerative medicine products.
Most importantly, the quality of the culture medium can influence
the quality and efficiency of cell culturing.
Click here to view the major deals in March 2018
(FREE Excel version available)
“The market for cell culture media is expanding following the dramatic growth in the demand for biopharmaceuticals centered around antibody drugs and the increasing need for treatments using cells, and its annual growth is expected to be approximately 10% going forward,” the company said in its official announcement.
Click here to view the major deals in March 2018
(FREE Excel version available)
Our View
The first quarter of 2018 has shown a relentless rise in dealmaking as an outcome of increased liquidity in the financial markets, new technological breakthroughs, dwindling new drug pipelines of major pharmaceutical companies, an aging population along with rising health consciousness among consumers is expected to further propel dealmaking in the world of pharmaceuticals.
With Pfizer always on the lookout, the upcoming quarter may just eclipse the records we’ve seen set in the first three months of the year.
Click here to view the major deals in March 2018
(FREE Excel version available)
Impressions: 2474
Each year,
the US Food and Drug Administration (FDA) approve hundreds
of new medications. A small subset of approvals, classified as novel drugs, are considered to
be truly innovative products that often help advance clinical care.
In 2015, the
FDA approved 45 novel drugs, an all-time record high. PharmaCompass has compiled a list of novel drugs approved by the FDA in 2015.The FDA also approved new dosage forms of existing products in the market (email us if you would like a copy), like the 3D printed version of anti-epilepsy drug, Spritam (Levetiracetam).
This week, PharmaCompass focuses on the new dosage
forms of existing drugs that got approved last year.
Modified blockbusters
Improving the delivery form of a blockbuster drug is something that not only helps patients but often successfully extends the patent life of the cash-generating drugs for Big Pharma. Here are some blockbuster drugs that saw their modified versions being launched in 2015:
Jadenu (deferasirox): With
almost a billion dollars in revenues in 2015, Exjade (deferasirox) was approved in 2005 as a
tablet for use in a suspension. Novartis, the innovator,
got approval in March 2015
for Jadenu, a once-daily oral tablet. Jadenu (deferasirox), a new formulation
of Exjade, is the only once-daily oral tablet for iron chelation. Jadenu has
simplified daily treatment administration for patients with chronic iron
overload.
Nexium
24HR (esomeprazole magnesium): Also
known as the Purple Pill, Nexium – Astra
Zeneca’s blockbuster drug for acid reflux that generated annual sales in America of more than US $ 3 billion – went generic in 2015. In order to extend Nexium’s market, Pfizer and AstraZeneca came together to promote an over-the-counter (OTC) version of Nexium. A capsule version of OTC Nexium was approved in 2014 and is known as
Nexium 24HR. Last year, the FDA granted approval to the tablet form of the
drug.
Iressa
(gefitinib): AstraZeneca re-introduced Iressa in
the US market in 2015. The
FDA had approved Gefitinib in May 2003 for non-small cell lung cancer. Approved
as a third-line therapy, in 2010 the FDA requested AstraZeneca to voluntarily withdraw Iressa tablets
from the market, as post-marketing studies had failed
to verify and confirm clinical benefit. Iressa (gefitinib) is now back in the US as a first-line therapy for a type of lung cancer. However, the patent protection is limited – only one listed patent in the Orange Book which expires next year, and five US Drug Master Files already submitted.
Onivyde (irinotecan): Liposomal formulation of anti-cancer
drugs have been in vogue for some time. Merrimack Pharmaceuticals got its novel encapsulation of Irinotecan in a liposomal formulation approved for the
treatment of patients with metastatic pancreatic cancer, sold under the brand
name Onivyde.
Vivlodex (meloxicam): In October 2015, the FDA approved 5 mg and 10 mg (administered once daily) doses of Vivlodex™ (meloxicam) capsules, a nonsteroidal anti-inflammatory drug (NSAID) used for the management of osteoarthritis pain. The previously approved doses for meloxicam capsules were 7.5mg and 15mg. Vivlodex uses a proprietary SoluMatrix Fine Particle Technology™, which contains meloxicam as submicron particles that are approximately 10 times smaller than their original size. The reduction in particle size provides an increased surface area, leading to faster dissolution.
Kalydeco (ivacaftor): A cystic fibrosis drug from Vertex Pharmaceuticals – Kalydeco – has been making headlines
because of its high price (more than US $ 300,000 a year). Price concerns
aside, 2015 saw the launch of a pediatric version of the drug as a ‘weight-based oral granule formulation of Kalydeco that can be mixed in soft foods or liquids’.
Extended release versions
Many of
the approvals granted by the FDA last year were to extended release
formulations (a pill formulated so that the drug is released slowly) of
existing drugs.
Kremers Urban’s
extended release version of Methylphenidate
capsules made headlines last year because of a reclassification of the drug by
the FDA. Under the new classification rating, methylphenidate hydrochloride extended-release tablets can be prescribed but may
not be automatically substituted for J&J’s reference drug Concerta (methylphenidate hydrochloride). Kremers Urban was almost sold last year. But due to this reclassification, investors aborted their US $ 1.53 billion buyout. Kremers Urban was later acquired by Lannett Company Inc.
In
addition, extended-release versions of Aspirin, Carbidopa/Levodopa, Paliperidone Palmitate, Tacrolimus
and Morphine Sulphate also received green signals for a market launch.
First generic opportunities
Last year, PharmaCompass
shared the names of some drugs which had no generic competition and were also
not protected by patents. (Read: “Litigation Free, first generic opportunities list”).
Deferiprone (a drug that chelates iron and is
used to treat iron overload in thalassemia major) met the criteria. But it still
has no generic competitor and is now available as a new dosage form.
Amedra Pharmaceuticals, now owned by Impax Laboratories, has enjoyed the rights to sell Albendazole tablets for almost two decades
without generic competition in the US. Albendazole is a medication used for the
treatment of a variety of parasitic worm infestations. In 2015, patients were
provided access to chewable tablets of Albendazole.
New combinations at work
The FDA also approved
multiple combination drugs where the individual active ingredients had been brought
to market previously.
Most of the combination drugs
approved belong to major pharma players like Novartis, Novo Nordisk, Bristol Myers etc.
Boehringer’s diabetes treatments – Jardiance (empagliflozin) – approved in 2014 and
Tradjenta (linagliptin) approved in 2011, were
combined and the combination drug product Glyxambi was approved in 2015. Another
combination of empagliflozin, with metformin – Synjardy – was also approved in August last
year.
Lesser known companies also
got combination drugs approved. UK-based
development company Vernalis got approval for its cold-cough treatment, Tuzistra XR – an extended release suspension of codeine polistirex and chlorpheniramine
polistirex.
Similarly, US-based biopharmaceutical startup, Spriaso LLC, also
working in the cold and cough therapeutic area, got an extended release tablet
containing codeine phosphate and chlorpheniramine maleate approved.
Symplmed, a company which is
developing various forms of Perindopril, got approval for Prestalia (a
combination of perindopril arginine and amlodipine besylate) for the
treatment of hypertension.
Our view
Each year, the FDA approves several
pharmaceutical drugs in order to improve patient care; and often versions of
these drugs are marketed and distributed across the globe.
PharmaCompass’ list of drugs approved in 2015 is now available – just email us for your copy.
Accelerate your drug development
PharmaCompass has also launched
the Drug Development Assistance tool on its platform.
Simply search for the drug or the active ingredient of your interest, click on the Drug Development icon on the left menu bar and you can see the inactive ingredients used to formulate
the various drug products approved in the United States.
Impressions: 5419